Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root
- PMID: 23940370
- PMCID: PMC3761638
- DOI: 10.1073/pnas.1308412110
Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root
Erratum in
- Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16283
Abstract
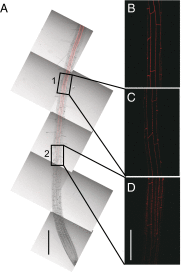
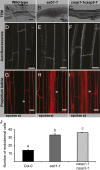
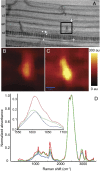
The endodermis acts as a "second skin" in plant roots by providing the cellular control necessary for the selective entry of water and solutes into the vascular system. To enable such control, Casparian strips span the cell wall of adjacent endodermal cells to form a tight junction that blocks extracellular diffusion across the endodermis. This junction is composed of lignin that is polymerized by oxidative coupling of monolignols through the action of a NADPH oxidase and peroxidases. Casparian strip domain proteins (CASPs) correctly position this biosynthetic machinery by forming a protein scaffold in the plasma membrane at the site where the Casparian strip forms. Here, we show that the dirigent-domain containing protein, enhanced suberin1 (ESB1), is part of this machinery, playing an essential role in the correct formation of Casparian strips. ESB1 is localized to Casparian strips in a CASP-dependent manner, and in the absence of ESB1, disordered and defective Casparian strips are formed. In addition, loss of ESB1 disrupts the localization of the CASP1 protein at the casparian strip domain, suggesting a reciprocal requirement for both ESB1 and CASPs in forming the casparian strip domain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Steudle E. The cohesion-tension mechanism and the acquisition of water by plant roots. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:847–875. - PubMed
-
- Steudle E. Water uptake by plant roots: An integration of views. Plant Soil. 2000;226:45–56.
-
- White PJ. The pathways of calcium movement to the xylem. J Exp Bot. 2001;52(358):891–899. - PubMed
-
- Geldner N. The endodermis. Annu Rev Plant Biol. 2013;64:531–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

