Green chemistry approach for the synthesis of biocompatible graphene
- PMID: 23940417
- PMCID: PMC3736970
- DOI: 10.2147/IJN.S45174
Green chemistry approach for the synthesis of biocompatible graphene
Abstract
Background: Graphene is a single-atom thick, two-dimensional sheet of hexagonally arranged carbon atoms isolated from its three-dimensional parent material, graphite. One of the most common methods for preparation of graphene is chemical exfoliation of graphite using powerful oxidizing agents. Generally, graphene is synthesized through deoxygenation of graphene oxide (GO) by using hydrazine, which is one of the most widespread and strongest reducing agents. Due to the high toxicity of hydrazine, it is not a promising reducing agent in large-scale production of graphene; therefore, this study focused on a green or sustainable synthesis of graphene and the biocompatibility of graphene in primary mouse embryonic fibroblast cells (PMEFs).
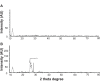
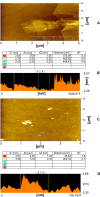
Methods: Here, we demonstrated a simple, rapid, and green chemistry approach for the synthesis of reduced GO (rGO) from GO using triethylamine (TEA) as a reducing agent and stabilizing agent. The obtained TEA reduced GO (TEA-rGO) was characterized by ultraviolet (UV)-visible absorption spectroscopy, X-ray diffraction (XRD), particle size dynamic light scattering (DLS), scanning electron microscopy (SEM), Raman spectroscopy, and atomic force microscopy (AFM).
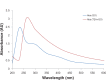
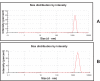
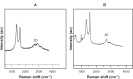
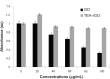
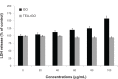
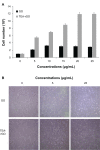
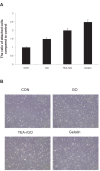
Results: The transition of graphene oxide to graphene was confirmed by UV-visible spectroscopy. XRD and SEM were used to investigate the crystallinity of graphene and the surface morphologies of prepared graphene respectively. The formation of defects further supports the functionalization of graphene as indicated in the Raman spectrum of TEA-rGO. Surface morphology and the thickness of the GO and TEA-rGO were analyzed using AFM. The presented results suggest that TEA-rGO shows significantly more biocompatibility with PMEFs cells than GO.
Conclusion: This is the first report about using TEA as a reducing as well as a stabilizing agent for the preparation of biocompatible graphene. The proposed safe and green method offers substitute routes for large-scale production of graphene for several biomedical applications.
Keywords: Raman spectroscopy; atomic force microscopy; graphene; graphene oxide; triethylamine; ultraviolet; visible spectroscopy.
Figures


References
-
- Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. - PubMed
-
- Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6(3):183–191. - PubMed
-
- Rao CN, Sood AK, Subrahmanyam KS, Govindaraj A. Graphene: the new two-dimensional nanomaterial. Angew Chem Int Ed Engl. 2009;48(42):7752–7777. - PubMed
-
- Dikin DA, Stankovich S, Zimney EJ, et al. Preparation and characterization of graphene oxide paper. Nature. 2007;448(7152):457–460. - PubMed
-
- Dreyer DR, Park S, Bielawski C, Ruoff RS. The chemistry of graphene oxide. Chem Soc Rev. 2009;39(1):228–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

