Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model
- PMID: 23940596
- PMCID: PMC3734284
- DOI: 10.1371/journal.pone.0070575
Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model
Abstract
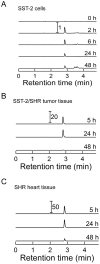
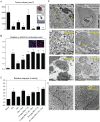
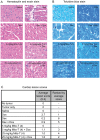
Several front-line chemotherapeutics cause mitochondria-derived, oxidative stress-mediated cardiotoxicity. Iron chelators and other antioxidants have not completely succeeded in mitigating this effect. One hindrance to the development of cardioprotectants is the lack of physiologically-relevant animal models to simultaneously study antitumor activity and cardioprotection. Therefore, we optimized a syngeneic rat model and examined the mechanisms by which oxidative stress affects outcome. Immune-competent spontaneously hypertensive rats (SHRs) were implanted with passaged, SHR-derived, breast tumor cell line, SST-2. Tumor growth and cytokine responses (IL-1A, MCP-1, TNF-α) were observed for two weeks post-implantation. To demonstrate the utility of the SHR/SST-2 model for monitoring both anticancer efficacy and cardiotoxicity, we tested cardiotoxic doxorubicin alone and in combination with an established cardioprotectant, dexrazoxane, or a nitroxide conjugated to a triphenylphosphonium cation, Mito-Tempol (4) [Mito-T (4)]. As predicted, tumor reduction and cardiomyopathy were demonstrated by doxorubicin. We confirmed mitochondrial accumulation of Mito-T (4) in tumor and cardiac tissue. Dexrazoxane and Mito-T (4) ameliorated doxorubicin-induced cardiomyopathy without altering the antitumor activity. Both agents increased the pro-survival autophagy marker LC3-II and decreased the apoptosis marker caspase-3 in the heart, independently and in combination with doxorubicin. Histopathology and transmission electron microscopy demonstrated apoptosis, autophagy, and necrosis corresponding to cytotoxicity in the tumor and cardioprotection in the heart. Changes in serum levels of 8-oxo-dG-modified DNA and total protein carbonylation corresponded to cardioprotective activity. Finally, 2D-electrophoresis/mass spectrometry identified specific serum proteins oxidized under cardiotoxic conditions. Our results demonstrate the utility of the SHR/SST-2 model and the potential of mitochondrially-directed agents to mitigate oxidative stress-induced cardiotoxicity. Our findings also emphasize the novel role of specific protein oxidation markers and autophagic mechanisms for cardioprotection.
Conflict of interest statement
Figures

References
-
- Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, et al. (2010) Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol 28: 3910–3916. - PubMed
-
- Mellor HR, Bell AR, Valentin JP, Roberts RR (2011) Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol Sci: 14–32. - PubMed
-
- Ferrans VJ, Clark JR, Zhang J, Yu ZX, Herman EH (1997) Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia 39: 928–937. - PubMed
-
- Levine RL, Stadtman ER (2001) Oxidative modification of proteins during aging. Exp Gerontol 36: 1495–1502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

