Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain
- PMID: 23940628
- PMCID: PMC3734263
- DOI: 10.1371/journal.pone.0070690
Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain
Abstract
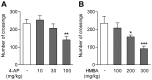
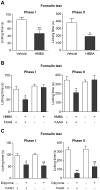
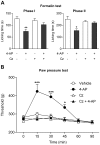
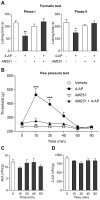
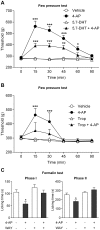
The discovery that paracetamol is metabolized to the potent TRPV1 activator N-(4-hydroxyphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (AM404) and that this metabolite contributes to paracetamol's antinociceptive effect in rodents via activation of TRPV1 in the central nervous system (CNS) has provided a potential strategy for developing novel analgesics. Here we validated this strategy by examining the metabolism and antinociceptive activity of the de-acetylated paracetamol metabolite 4-aminophenol and 4-hydroxy-3-methoxybenzylamine (HMBA), both of which may undergo a fatty acid amide hydrolase (FAAH)-dependent biotransformation to potent TRPV1 activators in the brain. Systemic administration of 4-aminophenol and HMBA led to a dose-dependent formation of AM404 plus N-(4-hydroxyphenyl)-9Z-octadecenamide (HPODA) and arvanil plus olvanil in the mouse brain, respectively. The order of potency of these lipid metabolites as TRPV1 activators was arvanil = olvanil>>AM404> HPODA. Both 4-aminophenol and HMBA displayed antinociceptive activity in various rodent pain tests. The formation of AM404, arvanil and olvanil, but not HPODA, and the antinociceptive effects of 4-aminophenol and HMBA were substantially reduced or disappeared in FAAH null mice. The activity of 4-aminophenol in the mouse formalin, von Frey and tail immersion tests was also lost in TRPV1 null mice. Intracerebroventricular injection of the TRPV1 blocker capsazepine eliminated the antinociceptive effects of 4-aminophenol and HMBA in the mouse formalin test. In the rat, pharmacological inhibition of FAAH, TRPV1, cannabinoid CB1 receptors and spinal 5-HT3 or 5-HT1A receptors, and chemical deletion of bulbospinal serotonergic pathways prevented the antinociceptive action of 4-aminophenol. Thus, the pharmacological profile of 4-aminophenol was identical to that previously reported for paracetamol, supporting our suggestion that this drug metabolite contributes to paracetamol's analgesic activity via activation of bulbospinal pathways. Our findings demonstrate that it is possible to construct novel antinociceptive drugs based on fatty acid conjugation as a metabolic pathway for the generation of TRPV1 modulators in the CNS.
Conflict of interest statement
Figures







References
-
- Högestätt ED, Jönsson BAG, Ermund A, Andersson DA, Björk H, et al. (2005) Conversion of acetaminophen to the bioactive N-acyl phenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem 280: 31405–31412. - PubMed
-
- Zygmunt PM, Chuang H, Movahed P, Julius D, Högestätt ED (2000) The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 396: 39–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

