Microglial P2Y12 deficiency/inhibition protects against brain ischemia
- PMID: 23940669
- PMCID: PMC3733797
- DOI: 10.1371/journal.pone.0070927
Microglial P2Y12 deficiency/inhibition protects against brain ischemia
Abstract
Objective: Microglia are among the first immune cells to respond to ischemic insults. Triggering of this inflammatory response may involve the microglial purinergic GPCR, P2Y12, activation via extracellular release of nucleotides from injured cells. It is also the inhibitory target of the widely used antiplatelet drug, clopidogrel. Thus, inhibiting this GPCR in microglia should inhibit microglial mediated neurotoxicity following ischemic brain injury.
Methods: Experimental cerebral ischemia was induced, in vitro with oxygen-glucose deprivation (OGD), or in vivo via bilateral common carotid artery occlusion (BCCAO). Genetic knock-down in vitro via siRNA, or in vivo P2Y12 transgenic mice (P2Y12-/- or P2Y12+/-), or in vivo treatment with clopidogrel, were used to manipulate the receptor. Neuron death, microglial activation, and microglial migration were assessed.
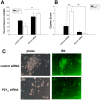
Results: The addition of microglia to neuron-astrocyte cultures increases neurotoxicity following OGD, which is mitigated by microglial P2Y12 deficiency (P<0.05). Wildtype microglia form clusters around these neurons following injury, which is also prevented in P2Y12 deficient microglia (P<0.01). P2Y12 knock-out microglia migrated less than WT controls in response to OGD-conditioned neuronal supernatant. P2Y12 (+/-) or clopidogrel treated mice subjected to global cerebral ischemia suffered less neuronal injury (P<0.01, P<0.001) compared to wild-type littermates or placebo treated controls. There were also fewer microglia surrounding areas of injury, and less activation of the pro-inflammatory transcription factor, nuclear factor Kappa B (NFkB).
Interpretation: P2Y12 participates in ischemia related inflammation by mediating microglial migration and potentiation of neurotoxicity. These data also suggest an additional anti-inflammatory, neuroprotective benefit of clopidogrel.
Conflict of interest statement
Figures




Similar articles
-
The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor.J Neuroinflammation. 2012 Sep 6;9:211. doi: 10.1186/1742-2094-9-211. J Neuroinflammation. 2012. PMID: 22950459 Free PMC article.
-
P2Y12 Receptor Modulates Sepsis-Induced Inflammation.Arterioscler Thromb Vasc Biol. 2016 May;36(5):961-71. doi: 10.1161/ATVBAHA.116.307401. Epub 2016 Apr 7. Arterioscler Thromb Vasc Biol. 2016. PMID: 27055904 Free PMC article.
-
Neuroprotective effects of the anti-inflammatory compound triflusal on ischemia-like neurodegeneration in mouse hippocampal slice cultures occur independent of microglia.Exp Neurol. 2009 Jul;218(1):11-23. doi: 10.1016/j.expneurol.2009.03.023. Epub 2009 Mar 31. Exp Neurol. 2009. PMID: 19341733
-
The role of P2Y12 receptor inhibition in ischemic stroke on microglia, platelets and vascular smooth muscle cells.J Thromb Thrombolysis. 2020 Nov;50(4):874-885. doi: 10.1007/s11239-020-02098-4. J Thromb Thrombolysis. 2020. PMID: 32248335 Review.
-
G-protein coupled purinergic P2Y12 receptor interacts and internalizes TauRD-mediated by membrane-associated actin cytoskeleton remodeling in microglia.Eur J Cell Biol. 2022 Apr;101(2):151201. doi: 10.1016/j.ejcb.2022.151201. Epub 2022 Jan 25. Eur J Cell Biol. 2022. PMID: 35101770 Review.
Cited by
-
Computational screening of biomarkers and potential drugs for arthrofibrosis based on combination of sequencing and large nature language model.J Orthop Translat. 2024 Jan 20;44:102-113. doi: 10.1016/j.jot.2023.11.002. eCollection 2024 Jan. J Orthop Translat. 2024. PMID: 38304615 Free PMC article.
-
Microglia is a key player in the reduction of stroke damage promoted by the new antithrombotic agent ticagrelor.J Cereb Blood Flow Metab. 2014 Jun;34(6):979-88. doi: 10.1038/jcbfm.2014.45. Epub 2014 Mar 19. J Cereb Blood Flow Metab. 2014. PMID: 24643079 Free PMC article.
-
Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway.J Neuroinflammation. 2015 Jun 26;12:124. doi: 10.1186/s12974-015-0344-2. J Neuroinflammation. 2015. PMID: 26112466 Free PMC article.
-
Ticagrelor promotes atherosclerotic plaque stability in a mouse model of advanced atherosclerosis.Drug Des Devel Ther. 2016 Aug 26;10:2691-9. doi: 10.2147/DDDT.S105718. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27616880 Free PMC article.
-
An Overview on the Differential Interplay Among Neurons-Astrocytes-Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia.Front Cell Neurosci. 2020 Nov 11;14:585833. doi: 10.3389/fncel.2020.585833. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33262692 Free PMC article. Review.
References
-
- Skaper SD (2011) Ion channels on microglia: therapeutic targets for neuroprotection. CNS Neurol Disord Drug Targets 10: 44–56. - PubMed
-
- Arbeloa J, Perez-Samartin A, Gottlieb M, Matute C (2012) P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis 45: 954–961. - PubMed
-
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte C, et al. (2006) P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 26: 974–982. - PubMed
-
- Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, et al. (2008) Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull 31: 1121–1130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

