Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy
- PMID: 23940688
- PMCID: PMC3733642
- DOI: 10.1371/journal.pone.0071045
Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy
Abstract
Background: Older patients are at high risk for experiencing Adverse Drug Events (ADEs) during hospitalization. To be able to reduce ADEs in these vulnerable patients, hospitals first need to measure the occurrence of ADEs, especially those that are preventable. However, data on preventable ADEs (pADEs) occurring during hospitalization in older patients are scarce, and no 'gold standard' for the identification of ADEs exists.
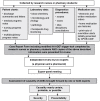
Methodology: The study was conducted in three hospitals in the Netherlands in 2007. ADEs were retrospectively identified by a team of experts using a comprehensive and structured patient chart review (PCR) combined with a trigger-tool as an aid. This ADE identification strategy was applied to a cohort of 250 older hospitalized patients. To estimate the intra- and inter-rater reliabilities, Cohen's kappa values were calculated.
Principal findings: In total, 118 ADEs were detected which occurred in 62 patients. This ADE yield was 1.1 to 2.7 times higher in comparison to other ADE studies in older hospitalized patients. Of the 118 ADEs, 83 (70.3%) were pADEs; 51 pADEs (43.2% of all ADEs identified) caused serious patient harm. Patient harm caused by ADEs resulted in various events. The overall intra-rater agreement of the developed strategy was substantial (κ = 0.74); the overall inter-rater agreement was only fair (κ = 0.24).
Conclusions/significance: The ADE identification strategy provided a detailed insight into the scope of ADEs occurring in older hospitalized patients, and showed that the majority of (serious) ADEs can be prevented. Several strategy related aspects, as well as setting/study specific aspects, may have contributed to the results gained. These aspects should be considered whenever ADE measurements need to be conducted. The results regarding pADEs can be used to design tailored interventions to effectively reduce harm caused by medication errors. Improvement of the inter-rater reliability of a PCR remains challenging.
Conflict of interest statement
Figures
References
-
- Zegers M, De Bruijne MC, Wagner C, Hoonhout LHF, Waaijman R, et al. (2009) Adverse events and potentially preventable deaths in Dutch hospitals: results of a retrospective patient record review study. Qual Saf Health Care 18: 297–302. - PubMed
-
- Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, et al. (1991) The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 324: 377–384. - PubMed
-
- Thürmann PA (2003) Detection of drug-related adverse events in hospitals. Expert Opin Drug Saf 2: 447–449. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical