Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model
- PMID: 23940699
- PMCID: PMC3733776
- DOI: 10.1371/journal.pone.0071116
Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model
Abstract
Background: New drugs and regimens with the potential to transform tuberculosis treatment are presently in early stage clinical trials.
Objective: The goal of the present study was to infer the required duration of these treatments.
Method: A meta-regression model was developed to predict relapse risk using treatment duration and month 2 sputum culture positive rate as predictors, based on published historical data from 24 studies describing 58 regimens in 7793 patients. Regimens in which rifampin was administered for the first 2 months but not subsequently were excluded. The model treated study as a random effect.
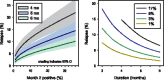
Results: The model predicted that new regimens of 4 or 5 months duration with rates of culture positivity after 2 months of 1% or 3%, would yield relapse rates of 4.0% or 4.1%, respectively. In both cases, the upper limit of the 2-sided 80% prediction interval for relapse for a hypothetical trial with 680 subjects per arm was <10%. Analysis using this model of published month 2 data for moxifloxacin-containing regimens indicated they would result in relapse rates similar to standard therapy only if administered for ≥5 months.
Conclusions: This model is proposed to inform the required duration of treatment of new TB regimens, potentially hastening their accelerated approval by several years.
Conflict of interest statement
Figures
References
-
- Wallis RS (2013) Sustainable tuberculosis drug development. Clin Infect Dis 56: 106–113. - PubMed
-
- Wallis RS, Wang C, Doherty TM, Onyebujoh P, Vahedi M, et al. (2010) Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 10: 68–69. - PubMed
-
- Fox W, Ellard GA, Mitchison DA (1999) Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 3: S231–S279. - PubMed
-
- Hong Kong Chest Service-British Medical Research Council (1978) Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis. First report. Am Rev Respir Dis 118: 219–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

