Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke
- PMID: 23940761
- PMCID: PMC3737090
- DOI: 10.1371/journal.pone.0071478
Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke
Abstract
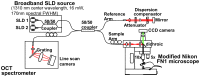
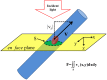
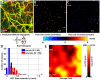
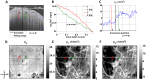
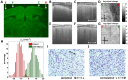
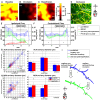
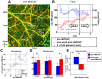
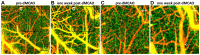
Progress in experimental stroke and translational medicine could be accelerated by high-resolution in vivo imaging of disease progression in the mouse cortex. Here, we introduce optical microscopic methods that monitor brain injury progression using intrinsic optical scattering properties of cortical tissue. A multi-parametric Optical Coherence Tomography (OCT) platform for longitudinal imaging of ischemic stroke in mice, through thinned-skull, reinforced cranial window surgical preparations, is described. In the acute stages, the spatiotemporal interplay between hemodynamics and cell viability, a key determinant of pathogenesis, was imaged. In acute stroke, microscopic biomarkers for eventual infarction, including capillary non-perfusion, cerebral blood flow deficiency, altered cellular scattering, and impaired autoregulation of cerebral blood flow, were quantified and correlated with histology. Additionally, longitudinal microscopy revealed remodeling and flow recovery after one week of chronic stroke. Intrinsic scattering properties serve as reporters of acute cellular and vascular injury and recovery in experimental stroke. Multi-parametric OCT represents a robust in vivo imaging platform to comprehensively investigate these properties.
Conflict of interest statement
Figures


References
-
- Schlaug G, Benfield A, Baird AE, Siewert B, Lovblad KO, et al. (1999) The ischemic penumbra - Operationally defined by diffusion and perfusion MRI. Neurology 53: 1528–1537. - PubMed
-
- Dunn AK, Bolay H, Moskowitz MA, Boas DA (2001) Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab 21: 195–201. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

