UDP induces intestinal epithelial migration via the P2Y6 receptor
- PMID: 23941325
- PMCID: PMC3799601
- DOI: 10.1111/bph.12334
UDP induces intestinal epithelial migration via the P2Y6 receptor
Abstract
Background and purpose: Extracellular nucleotides are released at high concentrations from damaged cells and function through P2 receptor activation. Intestinal epithelial restitution, which is defined as cell migration independent of cell proliferation, is an important initial step in the process of wound healing. In this study, we investigated the role of extracellular nucleotides in intestinal epithelial migratory responses.
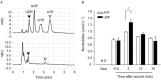
Experimental approach: Wound-healing and trans-well migration assays were performed with a rat intestinal epithelial cell line (IEC-6). The concentrations of extracellular nucleotides released from injured IEC-6 cells were measured by HPLC. TGF-β expression was assessed by RT-PCR and elisa.
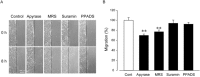
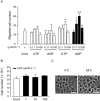
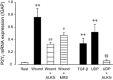
Key results: Scratching the monolayer of IEC-6 cells induced cell migration. Pretreatment with apyrase or MRS2578, a selective P2Y6 antagonist, inhibited the wound-induced cell migration. Among the cellular nucleotides, only ATP and uridine 5'-diphosphate (UDP) were detected in the culture medium after cell wounding. Exogenously applied UDP dose-dependently enhanced the migration more effectively than ATP but did not induce proliferation. In addition, cell wounding and UDP increased the expression of TGF-β, and both the wound-induced and UDP-enhanced migration were inhibited by MRS2578 or ALK5Inhibitor (ALK5i), a TGF-β receptor blocker. Furthermore, cell wounding and UDP stimulation up-regulated the expression of P2Y6 receptor mRNA, and this effect was suppressed by MRS2578 or ALK5i.
Conclusion and implications: Wound-induced UDP evokes intestinal epithelial restitution by activation of P2Y6 receptors, which mediates de novo synthesis of TGF-β. In addition, the expression of P2Y6 receptors is increased by cell wounding and UDP, which constitutes a positive-feedback loop for mucosal repair.
Keywords: P2Y6 receptor; TGF-β; UDP; extracellular nucleotides; intestinal epithelial migration.
© 2013 The British Pharmacological Society.
Figures




References
-
- Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. - PubMed
-
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545–564. - PubMed
-
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;276:11939–11948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

