Acute tumour response to the MEK1/2 inhibitor selumetinib (AZD6244, ARRY-142886) evaluated by non-invasive diffusion-weighted MRI
- PMID: 23942066
- PMCID: PMC3776979
- DOI: 10.1038/bjc.2013.456
Acute tumour response to the MEK1/2 inhibitor selumetinib (AZD6244, ARRY-142886) evaluated by non-invasive diffusion-weighted MRI
Abstract
Background: Non-invasive imaging biomarkers underpin the development of molecularly targeted anti-cancer drugs. This study evaluates tumour apparent diffusion coefficient (ADC), measured by diffusion-weighted magnetic resonance imaging (DW-MRI), as a biomarker of response to the MEK1/2 inhibitor selumetinib (AZD6244, ARRY-142886) in human tumour xenografts.
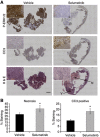
Methods: Nude mice bearing human BRAF(V600D) WM266.4 melanoma or BRAF(V600E) Colo205 colon carcinoma xenografts were treated for 4 days with vehicle or selumetinib. DW-MRI was performed before and 2 h after the last dose and excised tumours analysed for levels of phospho-ERK1/2, cleaved caspase 3 (CC3) and necrosis.
Results: Selumetinib treatment induced tumour stasis and reduced ERK1/2 phosphorylation in both WM266.4 and Colo205 tumour xenografts. Relative to day 0, mean tumour ADC was unchanged in the control groups but was significantly increased by up to 1.6-fold in selumetinib-treated WM266.4 and Colo205 tumours. Histological analysis revealed a significant increase in necrosis in selumetinib-treated WM266.4 and Colo205 xenografts and CC3 staining in selumetinib-treated Colo205 tumours relative to controls.
Conclusion: Changes in ADC following treatment with the MEK1/2 inhibitor selumetinib in responsive human tumour xenografts were concomitant with induction of tumour cell death. ADC may provide a useful non-invasive pharmacodynamic biomarker for early clinical assessment of response to selumetinib and other MEK-ERK1/2 signalling-targeted therapies.
Figures




References
-
- Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. - PMC - PubMed
-
- Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–692. - PubMed
-
- Banerji U, de BJ, Judson I, Kaye S, Workman P. Biomarkers in early clinical trials: the committed and the skeptics. Clin Cancer Res. 2008;14:2512–2514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

