Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury
- PMID: 23942364
- PMCID: PMC3851897
- DOI: 10.1038/jcbfm.2013.144
Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury
Abstract
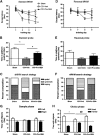
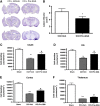
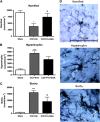
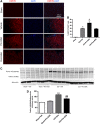
Geranylgeranylacetone (GGA) is an inducer of heat-shock protein 70 (HSP70) that has been used clinically for many years as an antiulcer treatment. It is centrally active after oral administration and is neuroprotective in experimental brain ischemia/stroke models. We examined the effects of single oral GGA before treatment (800 mg/kg, 48 hours before trauma) or after treatment (800 mg/kg, 3 hours after trauma) on long-term functional recovery and histologic outcomes after moderate-level controlled cortical impact, an experimental traumatic brain injury (TBI) model in mice. The GGA pretreatment increased the number of HSP70(+) cells and attenuated posttraumatic α-fodrin cleavage, a marker of apoptotic cell death. It also improved sensorimotor performance on a beam walk task; enhanced recovery of cognitive/affective function in the Morris water maze, novel object recognition, and tail-suspension tests; and improved outcomes using a composite neuroscore. Furthermore, GGA pretreatment reduced the lesion size and neuronal loss in the hippocampus, cortex, and thalamus, and decreased microglial activation in the cortex when compared with vehicle-treated TBI controls. Notably, GGA was also effective in a posttreatment paradigm, showing significant improvements in sensorimotor function, and reducing cortical neuronal loss. Given these neuroprotective actions and considering its longstanding clinical use, GGA should be considered for the clinical treatment of TBI.
Figures


Similar articles
-
Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma.J Cereb Blood Flow Metab. 2012 Jan;32(1):137-49. doi: 10.1038/jcbfm.2011.117. Epub 2011 Aug 10. J Cereb Blood Flow Metab. 2012. PMID: 21829212 Free PMC article.
-
Role of protein kinase C in neuroprotective effect of geranylgeranylacetone, a noninvasive inducing agent of heat shock protein, on delayed neuronal death caused by transient ischemia in rats.J Neurotrauma. 2006 Jul;23(7):1164-78. doi: 10.1089/neu.2006.23.1164. J Neurotrauma. 2006. PMID: 16866628
-
Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury.J Neurotrauma. 2014 Mar 1;31(5):476-86. doi: 10.1089/neu.2013.3135. Epub 2013 Dec 19. J Neurotrauma. 2014. PMID: 24070637 Free PMC article.
-
Neuroprotective profile of enoxaparin, a low molecular weight heparin, in in vivo models of cerebral ischemia or traumatic brain injury in rats: a review.CNS Drug Rev. 2002 Spring;8(1):1-30. doi: 10.1111/j.1527-3458.2002.tb00213.x. CNS Drug Rev. 2002. PMID: 12070524 Free PMC article. Review.
-
Animal models of traumatic brain injury.Nat Rev Neurosci. 2013 Feb;14(2):128-42. doi: 10.1038/nrn3407. Nat Rev Neurosci. 2013. PMID: 23329160 Free PMC article. Review.
Cited by
-
Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation.Pharmaceutics. 2020 Apr 30;12(5):414. doi: 10.3390/pharmaceutics12050414. Pharmaceutics. 2020. PMID: 32366047 Free PMC article.
-
Geranylgeranylacetone ameliorated ischemia/reperfusion induced-blood brain barrier breakdown through HSP70-dependent anti-apoptosis effect.Am J Transl Res. 2021 Jan 15;13(1):102-114. eCollection 2021. Am J Transl Res. 2021. PMID: 33527011 Free PMC article.
-
Role of a Heat Shock Transcription Factor and the Major Heat Shock Protein Hsp70 in Memory Formation and Neuroprotection.Cells. 2021 Jun 29;10(7):1638. doi: 10.3390/cells10071638. Cells. 2021. PMID: 34210082 Free PMC article. Review.
-
Geranylgeranylacetone Ameliorates Intestinal Radiation Toxicity by Preventing Endothelial Cell Dysfunction.Int J Mol Sci. 2017 Oct 7;18(10):2103. doi: 10.3390/ijms18102103. Int J Mol Sci. 2017. PMID: 28991157 Free PMC article.
-
Molecular chaperones and neuronal proteostasis.Semin Cell Dev Biol. 2015 Apr;40:142-52. doi: 10.1016/j.semcdb.2015.03.003. Epub 2015 Mar 12. Semin Cell Dev Biol. 2015. PMID: 25770416 Free PMC article. Review.
References
-
- Faul MXL, Wald MM, Coronado VG.Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deathsIn: (ed). Centers for Disease Control and Prevention National Center for Injury Prevention and Control Atlanta; 201013
-
- Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. - PubMed
-
- Yenari MA, Fink SL, Sun GH, Chang LK, Patel MK, Kunis DM, et al. Gene therapy with HSP72 is neuroprotective in rat models of stroke and epilepsy. Ann Neurol. 1998;44:584–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

