Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy
- PMID: 23942555
- PMCID: PMC3954983
- DOI: 10.1038/pr.2013.132
Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy
Abstract
Background: Neonates with hypoxic-ischemic encephalopathy (HIE) are at risk of cerebral blood flow dysregulation. Our objective was to describe the relationship between autoregulation and neurologic injury in HIE.
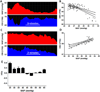
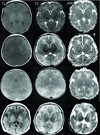
Methods: Neonates with HIE had autoregulation monitoring with the hemoglobin volume index (HVx) during therapeutic hypothermia, rewarming, and the first 6 h of normothermia. The 5-mm Hg range of mean arterial blood pressure (MAP) with best vasoreactivity (MAPOPT) was identified. The percentage of time spent with MAP below MAPOPT and deviation in MAP from MAPOPT were measured. Neonates received brain magnetic resonance imaging (MRI) 3-7 d after treatment. MRIs were coded as no, mild, or moderate/severe injury in five regions.
Results: HVx identified MAPOPT in 79% (19/24), 77% (17/22), and 86% (18/21) of the neonates during hypothermia, rewarming, and normothermia, respectively. Neonates with moderate/severe injury in paracentral gyri, white matter, basal ganglia, and thalamus spent a greater proportion of time with MAP below MAPOPT during rewarming than neonates with no or mild injury. Neonates with moderate/severe injury in paracentral gyri, basal ganglia, and thalamus had greater MAP deviation below MAPOPT during rewarming than neonates without injury.
Conclusion: Maintaining MAP within or above MAPOPT may reduce the risk of neurologic injuries in neonatal HIE.
Figures




References
-
- Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. 2008;199:587–595. - PubMed
-
- Zweifel C, Castellani G, Czosnyka M, et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma. 2010;27:1951–1958. - PubMed
-
- Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–2463. - PubMed
-
- Brady KM, Lee JK, Kibler KK, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108:1278–1283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

