Intestinal perfusion monitoring using photoplethysmography
- PMID: 23942635
- PMCID: PMC3739875
- DOI: 10.1117/1.JBO.18.8.087005
Intestinal perfusion monitoring using photoplethysmography
Abstract
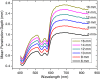
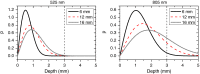
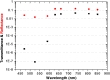
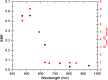
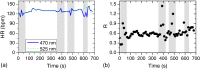
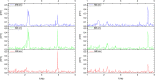
In abdominal trauma patients, monitoring intestinal perfusion and oxygen consumption is essential during the resuscitation period. Photoplethysmography is an optical technique potentially capable of monitoring these changes in real time to provide the medical staff with a timely and quantitative measure of the adequacy of resuscitation. The challenges for using optical techniques in monitoring hemodynamics in intestinal tissue are discussed, and the solutions to these challenges are presented using a combination of Monte Carlo modeling and theoretical analysis of light propagation in tissue. In particular, it is shown that by using visible wavelengths (i.e., 470 and 525 nm), the perfusion signal is enhanced and the background contribution is decreased compared with using traditional near-infrared wavelengths leading to an order of magnitude enhancement in the signal-to-background ratio. It was further shown that, using the visible wavelengths, similar sensitivity to oxygenation changes could be obtained (over 50% compared with that of near-infrared wavelengths). This is mainly due to the increased contrast between tissue and blood in that spectral region and the confinement of the photons to the thickness of the small intestine. Moreover, the modeling results show that the source to detector separation should be limited to roughly 6 mm while using traditional near-infrared light, with a few centimeters source to detector separation leads to poor signal-to-background ratio. Finally, a visible wavelength system is tested in an in vivo porcine study, and the possibility of monitoring intestinal perfusion changes is showed.
Figures








Similar articles
-
In vivo performance of a visible wavelength optical sensor for monitoring intestinal perfusion and oxygenation.J Biomed Opt. 2018 May;23(5):1-12. doi: 10.1117/1.JBO.23.5.055004. J Biomed Opt. 2018. PMID: 29777581 Free PMC article.
-
Monte Carlo Analysis of Optical Interactions in Reflectance and Transmittance Finger Photoplethysmography.Sensors (Basel). 2019 Feb 15;19(4):789. doi: 10.3390/s19040789. Sensors (Basel). 2019. PMID: 30769957 Free PMC article.
-
Algorithmic Principles of Remote PPG.IEEE Trans Biomed Eng. 2017 Jul;64(7):1479-1491. doi: 10.1109/TBME.2016.2609282. Epub 2016 Sep 13. IEEE Trans Biomed Eng. 2017. PMID: 28113245
-
[Quantitative perfusion imaging in magnetic resonance imaging].Radiologe. 2016 Feb;56(2):113-23. doi: 10.1007/s00117-015-0068-4. Radiologe. 2016. PMID: 26796337 Review. German.
-
Noninvasive monitoring by photoplethysmography.Clin Perinatol. 2012 Sep;39(3):573-83. doi: 10.1016/j.clp.2012.06.012. Clin Perinatol. 2012. PMID: 22954270 Review.
Cited by
-
In vivo performance of a visible wavelength optical sensor for monitoring intestinal perfusion and oxygenation.J Biomed Opt. 2018 May;23(5):1-12. doi: 10.1117/1.JBO.23.5.055004. J Biomed Opt. 2018. PMID: 29777581 Free PMC article.
-
A new photoplethysmographic device for continuous assessment of urethral mucosa perfusion: evaluation in a porcine model.J Clin Monit Comput. 2021 May;35(3):585-598. doi: 10.1007/s10877-020-00515-w. Epub 2020 May 2. J Clin Monit Comput. 2021. PMID: 32361961
-
Wireless monitoring of liver hemodynamics in vivo.PLoS One. 2014 Jul 14;9(7):e102396. doi: 10.1371/journal.pone.0102396. eCollection 2014. PLoS One. 2014. PMID: 25019160 Free PMC article.
-
In-silico and in-vitro investigation of a photonic monitor for intestinal perfusion and oxygenation.Biomed Opt Express. 2017 Jul 19;8(8):3714-3734. doi: 10.1364/BOE.8.003714. eCollection 2017 Aug 1. Biomed Opt Express. 2017. PMID: 28856045 Free PMC article.
-
A reusable hydrogel biosensor array with electrically responsive hydrogel interfaces for noninvasive locating of perforating arteries.Sci Adv. 2025 Jun 27;11(26):eadw6166. doi: 10.1126/sciadv.adw6166. Epub 2025 Jun 25. Sci Adv. 2025. PMID: 40561023 Free PMC article.
References
-
- Centers for Disease Control and Prevention, Injury in the United States: 2007 Chartbook, 2013, www.cdc.gov/traumacare (14 January 2013).
-
- WHO, Injuries and Violence: The Facts, World Health Organization, Geneva: (2010).
-
- Fries C. A., Midwinter M. J., “Trauma resuscitation and damage control surgery,” Surgery (Oxford) 28(11), 563–567 (2010).10.1016/j.mpsur.2010.08.002 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

