Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia
- PMID: 23943357
- PMCID: PMC4535913
- DOI: 10.1002/cncr.28318
Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia
Abstract
Background: The combination of fludarabine, cyclophosphamide, and rituximab (FCR) has produced improved response rates and a prolonged survival in patients with chronic lymphocytic leukemia (CLL). However, its therapeutic power is counterbalanced by significant hematologic toxicity. Persistent and new-onset cytopenia after the completion of FCR raise concern about disease recurrence, the development of therapy-related myeloid malignancies (TRMM), and infections.
Methods: A total of 207 patients with CLL who achieved complete response, complete response with incomplete bone marrow recovery, or nodular partial remission were analyzed after frontline FCR therapy.
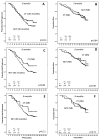
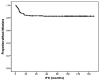
Results: Three months after the completion of therapy, 35% of patients had developed grade 2 to 4 cytopenia (according to Common Terminology Criteria for Adverse Events [version 4.0]). Factors found to be associated with cytopenia at 3 months after therapy were older age, advanced Rai stage disease, and lower baseline blood counts. Moreover, patients with cytopenia were less likely to have completed 6 courses of therapy with FCR. At 6 months and 9 months after therapy, the prevalence of grade 2 to 4 cytopenia was 24% and 12%, respectively. No differences in progression-free survival and overall survival were noted between cytopenic and noncytopenic patients or between patients with persistent and new-onset cytopenia. The prevalence of TRMM was 2.3% and did not differ significantly between cytopenic and noncytopenic patients or between those with persistent and new-onset disease. Late infections were more common in patients who were cytopenic at 9 months (38%) and were mostly bacterial (67%).
Conclusions: Cytopenia after the completion of therapy is a common complication of frontline FCR that improves over time, particularly for new-onset cases. The presence of persistent cytopenia (lasting up to 9 months after the completion of therapy) should not raise concern about CLL recurrence of the development of TRMM, but should encourage surveillance for bacterial infections for an additional 9 months.
Keywords: chronic lymphocytic leukemia; combination of fludarabine, cyclophosphamide, and rituximab; cytopenia; prognosis.
© 2013 American Cancer Society.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
Figures
Comment in
-
Reply to myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia.Cancer. 2014 Feb 1;120(3):452. doi: 10.1002/cncr.28442. Epub 2013 Oct 22. Cancer. 2014. PMID: 24151154 No abstract available.
-
Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia.Cancer. 2014 Feb 1;120(3):451-2. doi: 10.1002/cncr.28443. Epub 2013 Oct 22. Cancer. 2014. PMID: 24151174 No abstract available.
Similar articles
-
Myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia.Cancer. 2014 Feb 1;120(3):451-2. doi: 10.1002/cncr.28443. Epub 2013 Oct 22. Cancer. 2014. PMID: 24151174 No abstract available.
-
Reply to myelosuppression after frontline fludarabine, cyclophosphamide, and rituximab in patients with chronic lymphocytic leukemia: analysis of persistent and new-onset cytopenia.Cancer. 2014 Feb 1;120(3):452. doi: 10.1002/cncr.28442. Epub 2013 Oct 22. Cancer. 2014. PMID: 24151154 No abstract available.
-
Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial.Lancet Haematol. 2019 Aug;6(8):e419-e428. doi: 10.1016/S2352-3026(19)30104-8. Epub 2019 Jun 14. Lancet Haematol. 2019. PMID: 31208944 Free PMC article. Clinical Trial.
-
Fludarabine combination therapy for the treatment of chronic lymphocytic leukemia.Clin Lymphoma. 2002 Jun;3(1):26-35. doi: 10.3816/clm.2002.n.008. Clin Lymphoma. 2002. PMID: 12141952 Review.
-
Rituximab plus fludarabine and cyclophosphamide or other agents in chronic lymphocytic leukemia.Expert Rev Anticancer Ther. 2010 Oct;10(10):1529-43. doi: 10.1586/era.10.132. Expert Rev Anticancer Ther. 2010. PMID: 20942624 Review.
Cited by
-
Chronic Lymphocytic Leukemia and the Side Effects of Therapy: 2017 Review.Mo Med. 2017 May-Jun;114(3):195-198. Mo Med. 2017. PMID: 30228579 Free PMC article. Review.
-
Clinical utility and patient considerations in the use of ofatumumab in chronic lymphocytic leukemia.Biologics. 2015 Sep 18;9:75-86. doi: 10.2147/BTT.S60503. eCollection 2015. Biologics. 2015. PMID: 26425075 Free PMC article. Review.
-
Chimeric Antigen Receptor T-Cell Therapy and Hematopoiesis.Cells. 2023 Feb 7;12(4):531. doi: 10.3390/cells12040531. Cells. 2023. PMID: 36831198 Free PMC article. Review.
-
A phase II multi-center trial of pentostatin plus cyclophosphamide with ofatumumab in older previously untreated chronic lymphocytic leukemia patients.Haematologica. 2015 Dec;100(12):e501-4. doi: 10.3324/haematol.2015.132035. Epub 2015 Aug 20. Haematologica. 2015. PMID: 26294723 Free PMC article. Clinical Trial. No abstract available.
-
HBV-Associated Acute Liver Failure After Immunosuppression and Risk of Death.Clin Gastroenterol Hepatol. 2017 Jan;15(1):113-122. doi: 10.1016/j.cgh.2016.06.008. Epub 2016 Jun 13. Clin Gastroenterol Hepatol. 2017. PMID: 27311622 Free PMC article.
References
-
- Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. - PubMed
-
- Bottcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–988. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

