Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification
- PMID: 23943376
- PMCID: PMC4285748
- DOI: 10.1111/gcb.12351
Coralline algal structure is more sensitive to rate, rather than the magnitude, of ocean acidification
Abstract
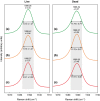
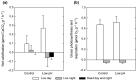
Marine pCO2 enrichment via ocean acidification (OA), upwelling and release from carbon capture and storage (CCS) facilities is projected to have devastating impacts on marine biomineralisers and the services they provide. However, empirical studies using stable endpoint pCO2 concentrations find species exhibit variable biological and geochemical responses rather than the expected negative patterns. In addition, the carbonate chemistry of many marine systems is now being observed to be more variable than previously thought. To underpin more robust projections of future OA impacts on marine biomineralisers and their role in ecosystem service provision, we investigate coralline algal responses to realistically variable scenarios of marine pCO2 enrichment. Coralline algae are important in ecosystem function; providing habitats and nursery areas, hosting high biodiversity, stabilizing reef structures and contributing to the carbon cycle. Red coralline marine algae were exposed for 80 days to one of three pH treatments: (i) current pH (control); (ii) low pH (7.7) representing OA change; and (iii) an abrupt drop to low pH (7.7) representing the higher rates of pH change observed at natural vent systems, in areas of upwelling and during CCS releases. We demonstrate that red coralline algae respond differently to the rate and the magnitude of pH change induced by pCO2 enrichment. At low pH, coralline algae survived by increasing their calcification rates. However, when the change to low pH occurred at a fast rate we detected, using Raman spectroscopy, weaknesses in the calcite skeleton, with evidence of dissolution and molecular positional disorder. This suggests that, while coralline algae will continue to calcify, they may be structurally weakened, putting at risk the ecosystem services they provide. Notwithstanding evolutionary adaptation, the ability of coralline algae to cope with OA may thus be determined primarily by the rate, rather than magnitude, at which pCO2 enrichment occurs.
Keywords: calcification; coralline algae; crustose coralline algae (CCA); maerl; ocean acidification; photosynthesis; raman; rate; respiration; rhodolith.
© 2013 John Wiley & Sons Ltd.
Figures

Similar articles
-
Major loss of coralline algal diversity in response to ocean acidification.Glob Chang Biol. 2021 Oct;27(19):4785-4798. doi: 10.1111/gcb.15757. Epub 2021 Jul 16. Glob Chang Biol. 2021. PMID: 34268846
-
Understanding coralline algal responses to ocean acidification: Meta-analysis and synthesis.Glob Chang Biol. 2022 Jan;28(2):362-374. doi: 10.1111/gcb.15899. Epub 2021 Oct 24. Glob Chang Biol. 2022. PMID: 34689395 Review.
-
Rhodoliths holobionts in a changing ocean: host-microbes interactions mediate coralline algae resilience under ocean acidification.BMC Genomics. 2018 Sep 24;19(1):701. doi: 10.1186/s12864-018-5064-4. BMC Genomics. 2018. PMID: 30249182 Free PMC article.
-
Coralline algae elevate pH at the site of calcification under ocean acidification.Glob Chang Biol. 2017 Oct;23(10):4245-4256. doi: 10.1111/gcb.13673. Epub 2017 Apr 2. Glob Chang Biol. 2017. PMID: 28370806
-
Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change.J Phycol. 2015 Feb;51(1):6-24. doi: 10.1111/jpy.12262. Epub 2015 Jan 23. J Phycol. 2015. PMID: 26986255 Free PMC article. Review.
Cited by
-
Environmental impacts on the structural integrity of British rhodoliths.Sci Rep. 2023 Aug 18;13(1):13473. doi: 10.1038/s41598-023-40292-5. Sci Rep. 2023. PMID: 37596363 Free PMC article.
-
Calcifying algae maintain settlement cues to larval abalone following algal exposure to extreme ocean acidification.Sci Rep. 2017 Jul 18;7(1):5774. doi: 10.1038/s41598-017-05502-x. Sci Rep. 2017. PMID: 28720836 Free PMC article.
-
Efficient carbon recycling between calcification and photosynthesis in red coralline algae.Biol Lett. 2024 Jun;20(6):20230598. doi: 10.1098/rsbl.2023.0598. Epub 2024 Jun 19. Biol Lett. 2024. PMID: 38889774 Free PMC article.
-
A possible link between coral reef success, crustose coralline algae and the evolution of herbivory.Sci Rep. 2020 Oct 20;10(1):17748. doi: 10.1038/s41598-020-73900-9. Sci Rep. 2020. PMID: 33082388 Free PMC article.
-
Baseline Assessment of Net Calcium Carbonate Accretion Rates on U.S. Pacific Reefs.PLoS One. 2015 Dec 7;10(12):e0142196. doi: 10.1371/journal.pone.0142196. eCollection 2015. PLoS One. 2015. PMID: 26641885 Free PMC article.
References
-
- Agnew DJ, Taylor AC. Effects of oxygen tension, temperature, salinity and humidity on the survival of two intertiday gammarid amphipods. Marine Ecology Progress Series. 1986;32:27–33.
-
- Anthony KRN, Kleypas J, Gattuso J-P. Coral reefs modify their seawater carbon chemistry - implications for impacts of ocean acidification. Global Change Biology. 2012;17:3655–3666.
-
- Biomaerl Team. Final Report (in 2 vols.), BIOMAERL Project. Scotland: P.G. Moore, University Marine Biological Station Millport; 1999. (Co-ordinator: pmoore@udcf.gla.ac.uk"). EC Contract No. MAS3-CT95-0020.
-
- Bischoff WD, Sharma SK, Mackenzie FT. Carbonate ion disorder in sythnetic and biogenic magnesian calcites: a Raman spectral study. American Mineralogist. 1985;70:581–589.
-
- Blackford J, Jones N, Proctor R, Holt J, Widdicombe S, Lowe D, Rees A. An initial assessment of the potential environmental impact of CO2 escape from marine carbon capture and storage systems. Proceedings of the Institution of Mechanical Engineers Part a-Journal of Power and Energy. 2009;223:269–280.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

