Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect
- PMID: 23943468
- PMCID: PMC3963803
- DOI: 10.1007/s13365-013-0190-x
Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect
Abstract
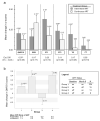
We evaluated factors associated with improvement in neurocognitive performance in 258 HIV-infected adults with baseline CD4 lymphocyte counts above 350 cells/mm³ randomized to intermittent, CD4-guided antiretroviral therapy (ART) (128 participants) versus continuous therapy (130) in the Neurology substudy of the Strategies for Management of Antiretroviral Therapy trial. Participants were enrolled in Australia, North America, Brazil, and Thailand, and neurocognitive performance was assessed by a five-test battery at baseline and month 6. The primary outcome was change in the quantitative neurocognitive performance z score (QNPZ-5), the average of the z scores of the five tests. Associations of the 6-month change in test scores with ART use, CD4 cell counts, HIV RNA levels, and other factors were determined using multiple regression models. At baseline, median age was 40 years, median CD4 cell count was 513 cells/mm³, 88 % had plasma HIV RNA ≤ 400 copies/mL, and mean QNPZ-5 was -0.68. Neurocognitive performance improved in both treatment groups by 6 months; QNPZ-5 scores increased by 0.20 and 0.13 in the intermittent and continuous ART groups, respectively (both P < 0.001 for increase and P = 0.26 for difference). ART was used on average for 3.6 and 5.9 out of the 6 months in the intermittent and continuous ART groups, respectively, but the increase in neurocognitive test scores could not be explained by ART use, changes in CD4, or plasma HIV RNA, which suggests a practice effect. The impact of a practice effect after 6 months emphasizes the need for a control group in HIV studies that measure intervention effects using neurocognitive tests similar to ours.
Figures

References
-
- Al-Khindi T, Zakzanis KK, van Gorp WG. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review of the literature. J Int Neuropsychol Soc. 2011;17:956–969. - PubMed
-
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. - PMC - PubMed
-
- Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, Phillips A, Porter K, Collaboration C. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neur. 2008;63:213–221. - PubMed
-
- Brouwers P, Hendricks M, Lietzau JA, Pluda JM, Mitsuya H, Broder S, Yarchoan R. Effect of combination therapy with zidovudine and didanosine on neuropsychological functioning in patients with symptomatic HIV disease: a comparison of simultaneous and alternating regimens. AIDS. 1997;11:59–66. - PubMed
-
- Carvalhal AS, Rourke SB, Belmonte-Abreu P, Correa J, Goldani LZ. Evaluation of neuropsychological performance of HIV-infected patients with minor motor cognitive dysfunction treated with highly active antiretroviral therapy. Infection. 2006;34:357–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01AI042170/AI/NIAID NIH HHS/United States
- R01 MH067751/MH/NIMH NIH HHS/United States
- R21NS0692/NS/NINDS NIH HHS/United States
- R01 MH062701/MH/NIMH NIH HHS/United States
- MH067751/MH/NIMH NIH HHS/United States
- U01 MH083545/MH/NIMH NIH HHS/United States
- P01 DA026134/DA/NIDA NIH HHS/United States
- U01AI46362/AI/NIAID NIH HHS/United States
- R01-R01MH081772/MH/NIMH NIH HHS/United States
- P01DA026134/DA/NIDA NIH HHS/United States
- U01-AI38858/AI/NIAID NIH HHS/United States
- U01-AI042170/AI/NIAID NIH HHS/United States
- MC_UU_12023/16/MRC_/Medical Research Council/United Kingdom
- U01 AI068641/AI/NIAID NIH HHS/United States
- R21 MH083520/MH/NIMH NIH HHS/United States
- R01 NS43103/NS/NINDS NIH HHS/United States
- U01-AI068641/AI/NIAID NIH HHS/United States
- U01 AI046362/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- MC_U122886352/MRC_/Medical Research Council/United Kingdom
- U01 AI068636/AI/NIAID NIH HHS/United States
- R01 MH081772/MH/NIMH NIH HHS/United States
- U01-AI4636/AI/NIAID NIH HHS/United States
- 1U01-AI068641/AI/NIAID NIH HHS/United States
- U01-AI46362/AI/NIAID NIH HHS/United States
- 1U01AI068636-01/AI/NIAID NIH HHS/United States
- U01 AI042170/AI/NIAID NIH HHS/United States
- R01 NS043103/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

