A computational model predicts that Gβγ acts at a cleft between channel subunits to activate GIRK1 channels
- PMID: 23943609
- PMCID: PMC4100999
- DOI: 10.1126/scisignal.2004075
A computational model predicts that Gβγ acts at a cleft between channel subunits to activate GIRK1 channels
Abstract
The atrial G protein (heterotrimeric guanine nucleotide-binding protein)-regulated inwardly rectifying K(+) (GIRK1 and GIRK4) heterotetrameric channels underlie the acetylcholine-induced K(+) current responsible for vagal inhibition of heart rate and are activated by the G protein βγ subunits (Gβγ). We used a multistage protein-protein docking approach with data from published structures of GIRK1 and Gβγ to generate an experimentally testable interaction model of Gβγ docked onto the cytosolic domains of the GIRK1 homotetramer. The model suggested a mechanism by which Gβγ promotes the open state of a specific cytosolic gate in the channel, the G loop gate. The predicted structure showed that the Gβ subunit interacts with the channel near the site of action for ethanol and stabilizes an intersubunit cleft formed by two loops (LM and DE) of adjacent channel subunits. Using a heterologous expression system, we disrupted the predicted GIRK1- and Gβγ-interacting residues by mutation of one protein and then rescued the regulatory activity by mutating reciprocal residues in the other protein. Disulfide cross-linking of channels and Gβγ with cysteine mutations at the predicted interacting residues yielded activated channels. The mechanism of Gβγ-induced activation of GIRK4 was distinct from GIRK1 homotetramers. However, GIRK1-GIRK4 heterotetrameric channels activated by Gβγ displayed responses indicating that the GIRK1 subunit dominated the response pattern. This work demonstrated that combining computational with experimental approaches is an effective method for elucidating interactions within protein complexes that otherwise might be challenging to decipher.
Conflict of interest statement
Figures

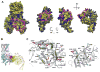
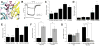

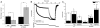
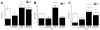
References
-
- Trautwein W, Dudel J. Zum mechanismus der membranwirkung des acetylcholin an der herzmuskelfaser. Pflügers Archiv European Journal of Physiology. 1958;266:324–334. - PubMed
-
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. - PubMed
-
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G Protein Structure, Function, and Regulation. Endocrine Reviews. 2003;24:765–781. - PubMed
-
- Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

