Activation of protein kinase C-mitogen-activated protein kinase signaling in response to inositol starvation triggers Sir2p-dependent telomeric silencing in yeast
- PMID: 23943620
- PMCID: PMC3784701
- DOI: 10.1074/jbc.M113.493072
Activation of protein kinase C-mitogen-activated protein kinase signaling in response to inositol starvation triggers Sir2p-dependent telomeric silencing in yeast
Abstract
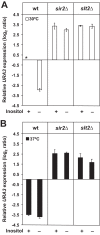
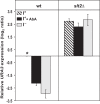
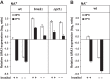
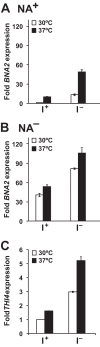
Depriving wild type yeast of inositol, a soluble precursor for phospholipid, phosphoinositide, and complex sphingolipid synthesis, activates the protein kinase C (PKC)-MAPK signaling pathway, which plays a key role in the activation of NAD(+)-dependent telomeric silencing. We now report that triggering PKC-MAPK signaling by inositol deprivation or by blocking inositol-containing sphingolipid synthesis with aureobasidin A results in increased telomeric silencing regulated by the MAPK, Slt2p, and the NAD(+)-dependent deacetylase, Sir2p. Consistent with the dependence on NAD(+) in Sir2p-regulated silencing, we found that inositol depletion induces the expression of BNA2, which is required for the de novo synthesis of NAD(+). Moreover, telomeric silencing is greatly reduced in bna2Δ and npt1Δ mutants, which are defective in de novo and salvage pathways for NAD(+) synthesis, respectively. Surprisingly, however, omitting nicotinic acid from the growth medium, which reduces cellular NAD(+) levels, leads to increased telomeric silencing in the absence of inositol and/or at high temperature. This increase in telomeric silencing in response to inositol starvation is correlated to chronological life span extension but is Sir2p-independent. We conclude that activation of the PKC-MAPK signaling by interruption of inositol sphingolipid synthesis leads to increased Sir2p-dependent silencing and is dependent upon the de novo and salvage pathways for NAD(+) synthesis but is not correlated with cellular NAD(+) levels.
Keywords: Inositol Phospholipid; NAD Biosynthesis; Protein Kinase C (PKC); Sirtuins; Sphingolipid.
Figures


Similar articles
-
Telomeric and rDNA silencing in Saccharomyces cerevisiae are dependent on a nuclear NAD(+) salvage pathway.Genetics. 2002 Mar;160(3):877-89. doi: 10.1093/genetics/160.3.877. Genetics. 2002. PMID: 11901108 Free PMC article.
-
Saccharomyces cerevisiae Esc2p interacts with Sir2p through a small ubiquitin-like modifier (SUMO)-binding motif and regulates transcriptionally silent chromatin in a locus-dependent manner.J Biol Chem. 2010 Mar 5;285(10):7525-36. doi: 10.1074/jbc.M109.016360. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048165 Free PMC article.
-
Pnc1p-mediated nicotinamide clearance modifies the epigenetic properties of rDNA silencing in Saccharomyces cerevisiae.Genetics. 2008 Oct;180(2):797-810. doi: 10.1534/genetics.108.091090. Epub 2008 Sep 9. Genetics. 2008. PMID: 18780747 Free PMC article.
-
Sound silencing: the Sir2 protein and cellular senescence.Bioessays. 2001 Apr;23(4):327-32. doi: 10.1002/bies.1047. Bioessays. 2001. PMID: 11268038 Review.
-
Regulation of yeast sirtuins by NAD(+) metabolism and calorie restriction.Biochim Biophys Acta. 2010 Aug;1804(8):1567-75. doi: 10.1016/j.bbapap.2009.09.030. Epub 2009 Oct 8. Biochim Biophys Acta. 2010. PMID: 19818879 Free PMC article. Review.
Cited by
-
Evolution of Distinct Responses to Low NAD+ Stress by Rewiring the Sir2 Deacetylase Network in Yeasts.Genetics. 2020 Apr;214(4):855-868. doi: 10.1534/genetics.120.303087. Epub 2020 Feb 18. Genetics. 2020. PMID: 32071196 Free PMC article.
-
Sphingolipids and mitochondrial function, lessons learned from yeast.Microb Cell. 2014 Jun 25;1(7):210-224. doi: 10.15698/mic2014.07.156. Microb Cell. 2014. PMID: 28357246 Free PMC article. Review.
-
Vps74 Connects the Golgi Apparatus and Telomeres in Saccharomyces cerevisiae.G3 (Bethesda). 2018 May 4;8(5):1807-1816. doi: 10.1534/g3.118.200172. G3 (Bethesda). 2018. PMID: 29593073 Free PMC article.
-
The capacity of Aspergillus niger to sense and respond to cell wall stress requires at least three transcription factors: RlmA, MsnA and CrzA.Fungal Biol Biotechnol. 2014 Dec 1;1:5. doi: 10.1186/s40694-014-0005-8. eCollection 2014. Fungal Biol Biotechnol. 2014. PMID: 28955447 Free PMC article.
-
Cell Wall-Associated Virulence Factors Contribute to Increased Resilience of Old Cryptococcus neoformans Cells.Front Microbiol. 2019 Nov 7;10:2513. doi: 10.3389/fmicb.2019.02513. eCollection 2019. Front Microbiol. 2019. PMID: 31787940 Free PMC article.
References
-
- Jesch S. A., Liu P., Zhao X., Wells M. T., Henry S. A. (2006) Multiple endoplasmic reticulum-to-nucleus signaling pathways coordinate phospholipid metabolism with gene expression by distinct mechanisms. J. Biol. Chem. 281, 24070–24083 - PubMed
-
- Santiago T. C., Mamoun C. B. (2003) Genome expression analysis in yeast reveals novel transcriptional regulation by inositol and choline and new regulatory functions for Opi1p, Ino2p, and Ino4p. J. Biol. Chem. 278, 38723–38730 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

