Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the conversion of white-to-brown adipocytes
- PMID: 23943621
- PMCID: PMC3784732
- DOI: 10.1074/jbc.M113.468603
Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the conversion of white-to-brown adipocytes
Abstract
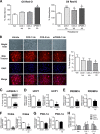
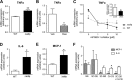
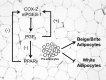
Peroxisome proliferator-activated receptor γ (PPARγ) is a ligand-activated nuclear receptor and a master regulator of adipogenesis. Microsomal prostaglandin E (PGE) synthase-1 (mPGES-1) is an inducible enzyme that couples with cyclooxygenase-2 for the biosynthesis of PGE2. In this study we demonstrate the existence of a coordinate functional interaction between PPARγ and mPGES-1 in controlling the process of pre-adipocyte differentiation in white adipose tissue (WAT). Adipocyte-specific PPARγ knock-out mice carrying an aP2 promoter-driven Cre recombinase transgene showed a blunted response to the adipogenic effects of a high fat diet. Pre-adipocytes from these knock-out mice showed loss of PPARγ and were resistant to rosiglitazone-induced WAT differentiation. In parallel, WAT from these mice showed increased expression of uncoupling protein 1, a mitochondrial enzyme that dissipates chemical energy as heat. Adipose tissue from mice lacking PPARγ also showed mPGES-1 up-regulation and increased PGE2 levels. In turn, PGE2 suppressed PPARγ expression and blocked rosiglitazone-induced pre-adipocyte differentiation toward white adipocytes while directly elevating uncoupling protein 1 expression and pre-adipocyte differentiation into mature beige/brite adipocytes. Consistently, pharmacological mPGES-1 inhibition directed pre-adipocyte differentiation toward white adipocytes while suppressing differentiation into beige/brite adipocytes. This browning effect was reproduced in knockdown experiments using a siRNA directed against mPGES-1. The effects of PGE2 on pre-adipocyte differentiation were not seen in mice lacking PPARγ in adipose tissue and were not mirrored by other eicosanoids (i.e. leukotriene B4). Taken together, these findings identify PGE2 as a key regulator of white-to-brown adipogenesis and suggest the existence of a coordinate regulation of adipogenesis between PPARγ and mPGES-1.
Keywords: Adipose Tissue; Beige Adipocytes; Cyclooxygenase (COX) Pathway; Eicosanoid; Metabolic Diseases; PPARγ; Pre-adipocytes; Prostaglandins; mPGES-1.
Figures




Similar articles
-
Down-regulation of microsomal prostaglandin E2 synthase-1 in adipose tissue by high-fat feeding.Obesity (Silver Spring). 2007 Jan;15(1):60-8. doi: 10.1038/oby.2007.514. Obesity (Silver Spring). 2007. PMID: 17228032
-
Indomethacin promotes browning and brown adipogenesis in both murine and human fat cells.Pharmacol Res Perspect. 2020 Jun;8(3):e00592. doi: 10.1002/prp2.592. Pharmacol Res Perspect. 2020. PMID: 32430973 Free PMC article.
-
Microsomal prostaglandin E synthase-1 deficiency is associated with elevated peroxisome proliferator-activated receptor gamma: regulation by prostaglandin E2 via the phosphatidylinositol 3-kinase and Akt pathway.J Biol Chem. 2007 Feb 23;282(8):5356-66. doi: 10.1074/jbc.M610153200. Epub 2006 Dec 22. J Biol Chem. 2007. PMID: 17186945
-
Brown vs white adipocytes: the PPARgamma coregulator story.FEBS Lett. 2010 Aug 4;584(15):3250-9. doi: 10.1016/j.febslet.2010.06.035. Epub 2010 Jun 30. FEBS Lett. 2010. PMID: 20600006 Review.
-
White, brown, beige/brite: different adipose cells for different functions?Endocrinology. 2013 Sep;154(9):2992-3000. doi: 10.1210/en.2013-1403. Epub 2013 Jun 19. Endocrinology. 2013. PMID: 23782940 Review.
Cited by
-
Lipokines and Thermogenesis.Endocrinology. 2019 Oct 1;160(10):2314-2325. doi: 10.1210/en.2019-00337. Endocrinology. 2019. PMID: 31504387 Free PMC article. Review.
-
Lipolysis-derived linoleic acid drives beige fat progenitor cell proliferation.Dev Cell. 2022 Dec 5;57(23):2623-2637.e8. doi: 10.1016/j.devcel.2022.11.007. Dev Cell. 2022. PMID: 36473459 Free PMC article.
-
The ω6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway.Mol Metab. 2014 Sep 16;3(9):834-47. doi: 10.1016/j.molmet.2014.09.003. eCollection 2014 Dec. Mol Metab. 2014. PMID: 25506549 Free PMC article.
-
Calycosin attenuates renal ischemia/reperfusion injury by suppressing NF-κB mediated inflammation via PPARγ/EGR1 pathway.Front Pharmacol. 2022 Oct 7;13:970616. doi: 10.3389/fphar.2022.970616. eCollection 2022. Front Pharmacol. 2022. PMID: 36278223 Free PMC article.
-
Altered adipocyte differentiation and unbalanced autophagy in type 2 Familial Partial Lipodystrophy: an in vitro and in vivo study of adipose tissue browning.Exp Mol Med. 2019 Aug 2;51(8):1-17. doi: 10.1038/s12276-019-0289-0. Exp Mol Med. 2019. PMID: 31375660 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

