Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles
- PMID: 23943763
- PMCID: PMC3747587
- DOI: 10.1128/mBio.00524-13
Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles
Abstract
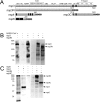
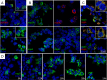
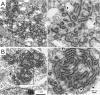
Coronaviruses (CoV), like other positive-stranded RNA viruses, redirect and rearrange host cell membranes for use as part of the viral genome replication and transcription machinery. Specifically, coronaviruses induce the formation of double-membrane vesicles in infected cells. Although these double-membrane vesicles have been well characterized, the mechanism behind their formation remains unclear, including which viral proteins are responsible. Here, we use transfection of plasmid constructs encoding full-length versions of the three transmembrane-containing nonstructural proteins (nsps) of the severe acute respiratory syndrome (SARS) coronavirus to examine the ability of each to induce double-membrane vesicles in tissue culture. nsp3 has membrane disordering and proliferation ability, both in its full-length form and in a C-terminal-truncated form. nsp3 and nsp4 working together have the ability to pair membranes. nsp6 has membrane proliferation ability as well, inducing perinuclear vesicles localized around the microtubule organizing center. Together, nsp3, nsp4, and nsp6 have the ability to induce double-membrane vesicles that are similar to those observed in SARS coronavirus-infected cells. This activity appears to require the full-length form of nsp3 for action, as double-membrane vesicles were not seen in cells coexpressing the C-terminal truncation nsp3 with nsp4 and nsp6.
Importance: Although the majority of infections caused by coronaviruses in humans are relatively mild, the SARS outbreak of 2002 to 2003 and the emergence of the human coronavirus Middle Eastern respiratory syndrome (MERS-CoV) in 2012 highlight the ability of these viruses to cause severe pathology and fatality. Insight into the molecular biology of how coronaviruses take over the host cell is critical for a full understanding of any known and possible future outbreaks caused by these viruses. Additionally, since membrane rearrangement is a tactic used by all known positive-sense single-stranded RNA viruses, this work adds to that body of knowledge and may prove beneficial in the development of future therapies not only for human coronavirus infections but for other pathogens as well.
Figures



References
-
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguière AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Müller S, Rickerts V, Stürmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967–1976 - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, Rollin PE, Dowell SF, Ling AE, Humphrey CD, Shieh WJ, Guarner J, Paddock CD, Rota P, Fields B, DeRisi J, Yang JY, Cox N, Hughes JM, LeDuc JW, Bellini WJ, Anderson LJ, SARS Working Group 2003. novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953–1966 - PubMed
-
- Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, Stellberger T, von Dall’armi E, Herzog P, Kallies S, Niemeyer D, Ditt V, Kuri T, Züst R, Pumpor K, Hilgenfeld R, Schwarz F, Zimmer R, Steffen I, Weber F, Thiel V, Herrler G, Thiel HJ, Schwegmann-Wessels C, Pöhlmann S, Haas J, Drosten C, von Brunn A. 2011. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLOS Pathog. 7:e1002331 http://dx.doi.org/10.1371/journal.ppat.1002331 - PMC - PubMed
-
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ, National Microbiology Laboratory. Canada. Canadian Severe Acute Respiratory Syndrome Study Team 2003. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 348:1995–2005 - PubMed
-
- Centers for Disease Control and Prevention. CDC 2003. Update: outbreak of severe acute respiratory syndrome—worldwide, 2003. MMWR Morb. Mortal. Wkly. Rep. 52:269–272 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
