Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation
- PMID: 23943825
- PMCID: PMC3805172
- DOI: 10.1093/cid/cit538
Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation
Abstract
Background: Bone mineral density (BMD) decreases 2%-6% in the 2 years after antiretroviral therapy (ART) initiation. Pre-ART immune deficiency and early immune recovery may contribute to this loss.
Methods: We pooled data from 3 studies of ART initiation in treatment-naive patients in which serial whole-body dual-energy X-ray absorptiometry scans were performed. We used linear regression to evaluate effects of baseline CD4(+) and 16-week CD4(+) change (both absolute and relative) on 96-week total BMD change from baseline. We performed multivariable linear regression to assess associations between baseline variables of age, sex, race/ethnicity, body mass index (BMI), hepatitis C status, parent study, human immunodeficiency virus type 1 (HIV-1) RNA level, and assignment to a protease inhibitor (PI)- or tenofovir-containing regimen on 96-week total BMD change.
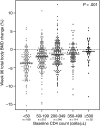
Results: The included 796 subjects had mean 96-week total BMD loss of 2.0%. In multivariable analysis, baseline CD4(+) cell count was significantly associated with 96-week BMD loss; individuals with baseline CD4(+) <50 cells/µL lost significantly more BMD compared to those with CD4(+) ≥500 cells/µL. A greater relative, but not absolute, 16-week increase in CD4(+) count was significantly associated with greater declines in BMD, but not after controlling for baseline CD4(+) count. In multivariable analysis, older age, female sex, lower BMI, higher HIV-1 RNA levels, and PI and tenofovir assignment were also associated with greater BMD decline.
Conclusions: Low pretreatment CD4(+) count, but not greater CD4(+) count increase, is a strong and independent risk factor for bone loss after ART initiation. ART initiation at higher CD4(+) counts may reduce the burden of osteoporosis and fragility fractures.
Keywords: HIV infections; administration and dosage; anti-HIV agents; bone density; drug therapy/virology.
Figures
References
-
- Weber R, Smith C D:A:D Study Group. Trends over time in underlying causes of death in the D:A:D study from 1999 to 2011. International AIDS Conference,; 22–27 July 2012; Washington, DC,. Abstract THAB0304.
-
- Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–74. - PubMed
-
- McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–801. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1AI069434/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- R01 AI065348/AI/NIAID NIH HHS/United States
- AI065348/AI/NIAID NIH HHS/United States
- AI068634/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- K24 AI064086/AI/NIAID NIH HHS/United States
- AI36214/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- AI064086/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- AI38855/AI/NIAID NIH HHS/United States
- AI069432/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous