Strigolactones stimulate internode elongation independently of gibberellins
- PMID: 23943865
- PMCID: PMC3793021
- DOI: 10.1104/pp.113.220541
Strigolactones stimulate internode elongation independently of gibberellins
Abstract
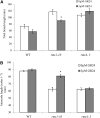
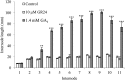
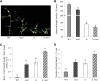
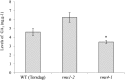
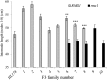
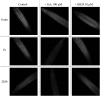
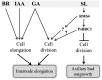
Strigolactone (SL) mutants in diverse species show reduced stature in addition to their extensive branching. Here, we show that this dwarfism in pea (Pisum sativum) is not attributable to the strong branching of the mutants. The continuous supply of the synthetic SL GR24 via the root system using hydroponics can restore internode length of the SL-deficient rms1 mutant but not of the SL-response rms4 mutant, indicating that SLs stimulate internode elongation via RMS4. Cytological analysis of internode epidermal cells indicates that SLs control cell number but not cell length, suggesting that SL may affect stem elongation by stimulating cell division. Consequently, SLs can repress (in axillary buds) or promote (in the stem) cell division in a tissue-dependent manner. Because gibberellins (GAs) increase internode length by affecting both cell division and cell length, we tested if SLs stimulate internode elongation by affecting GA metabolism or signaling. Genetic analyses using SL-deficient and GA-deficient or DELLA-deficient double mutants, together with molecular and physiological approaches, suggest that SLs act independently from GAs to stimulate internode elongation.
Figures




References
-
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GT, Genschik P. (2009) Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol 19: 1188–1193 - PubMed
-
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

