Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease
- PMID: 23945235
- PMCID: PMC3754239
- DOI: 10.1172/JCI65570
Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease
Erratum in
- J Clin Invest. 2013 Dec 2;123(12):5410
Abstract
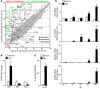
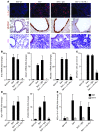
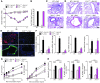
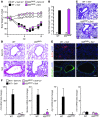
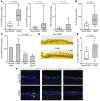
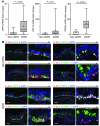
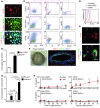
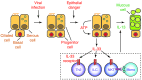
Chronic obstructive lung disease is characterized by persistent abnormalities in epithelial and immune cell function that are driven, at least in part, by infection. Analysis of parainfluenza virus infection in mice revealed an unexpected role for innate immune cells in IL-13-dependent chronic lung disease, but the upstream driver for the immune axis in this model and in humans with similar disease was undefined. We demonstrate here that lung levels of IL-33 are selectively increased in postviral mice with chronic obstructive lung disease and in humans with very severe chronic obstructive pulmonary disease (COPD). In the mouse model, IL-33/IL-33 receptor signaling was required for Il13 and mucin gene expression, and Il33 gene expression was localized to a virus-induced subset of airway serous cells and a constitutive subset of alveolar type 2 cells that are both linked conventionally to progenitor function. In humans with COPD, IL33 gene expression was also associated with IL13 and mucin gene expression, and IL33 induction was traceable to a subset of airway basal cells with increased capacities for pluripotency and ATP-regulated release of IL-33. Together, these findings provide a paradigm for the role of the innate immune system in chronic disease based on the influence of long-term epithelial progenitor cells programmed for excess IL-33 production.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL029594/HL/NHLBI NIH HHS/United States
- R01 HL073159/HL/NHLBI NIH HHS/United States
- P50-HL107183/HL/NHLBI NIH HHS/United States
- R01 HL121791/HL/NHLBI NIH HHS/United States
- U19 AI070489/AI/NIAID NIH HHS/United States
- P50 HL107183/HL/NHLBI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- U19-AI070489/AI/NIAID NIH HHS/United States
- U54-AI05160/AI/NIAID NIH HHS/United States
- K08 HL083095/HL/NHLBI NIH HHS/United States
- R01-HL073159/HL/NHLBI NIH HHS/United States
- T32 HL007317/HL/NHLBI NIH HHS/United States
- K08-HL083095/HL/NHLBI NIH HHS/United States
- P01-HL29594/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

