Schnurri-3 regulates ERK downstream of WNT signaling in osteoblasts
- PMID: 23945236
- PMCID: PMC3754267
- DOI: 10.1172/JCI69443
Schnurri-3 regulates ERK downstream of WNT signaling in osteoblasts
Abstract
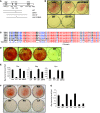
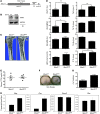
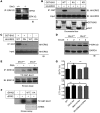
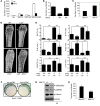
Mice deficient in Schnurri-3 (SHN3; also known as HIVEP3) display increased bone formation, but harnessing this observation for therapeutic benefit requires an improved understanding of how SHN3 functions in osteoblasts. Here we identified SHN3 as a dampener of ERK activity that functions in part downstream of WNT signaling in osteoblasts. A D-domain motif within SHN3 mediated the interaction with and inhibition of ERK activity and osteoblast differentiation, and knockin of a mutation in Shn3 that abolishes this interaction resulted in aberrant ERK activation and consequent osteoblast hyperactivity in vivo. Additionally, in vivo genetic interaction studies demonstrated that crossing to Lrp5(-/-) mice partially rescued the osteosclerotic phenotype of Shn3(-/-) mice; mechanistically, this corresponded to the ability of SHN3 to inhibit ERK-mediated suppression of GSK3β. Inducible knockdown of Shn3 in adult mice resulted in a high-bone mass phenotype, providing evidence that transient blockade of these pathways in adults holds promise as a therapy for osteoporosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

