Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure
- PMID: 23945503
- PMCID: PMC4016763
- DOI: 10.1097/QAD.0b013e32835f2b49
Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure
Abstract
Objective: To investigate the concentration of the integrase strand inhibitor raltegravir (RAL) throughout gastrointestinal (GI) tissue, especially gut-associated lymphoid tissue (GALT), as an adjunct to current prevention and cure strategies.
Design: Open-label pharmacokinetic (PK) study.
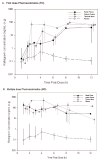
Methods: HIV-negative men received RAL 400 mg twice daily for 7 days. Seven blood plasma specimens were collected over 12-h intervals; timed tissue specimens from terminal ileum, splenic flexure, and rectum were also obtained by colonoscopy following the first dose and on day 7 [multiple dose (MD)]. RAL concentrations were measured by validated LC-MS assay with 1 ng/ml lower limit of detection. Data were analyzed by noncompartmental methods (WinNonlin 6). Tissue exposures are reported as composite medians and tissue density of 1.04 g/ml is assumed for comparisons.
Results: Fourteen men completed evaluations. Median (range) age was 24 (19-49) years and BMI 25 (19-31) kg/m². After the first dose, area under the time-concentration curve (AUC)(0-12h) was highest in the terminal ileum (594 μg*h/ml). Exposures were 160, 68 and 39-fold greater than blood plasma at the terminal ileum, splenic flexure and rectum, respectively. After multiple doses, exposure was highest at the splenic flexure (2240 μg*h/ml); exposure at the terminal ileum and rectum were equivalent (both 788 μg*h/ml). Following multiple doses, exposures were 160 to 650-fold greater than blood plasma throughout the colon.
Conclusion: RAL rapidly disseminates into GI tissue and concentrations remain significantly higher than blood plasma. RAL exposure in GI tissue remains higher than any antiretroviral investigated to date. These data suggest that RAL should result in full suppression of viral replication in GI tissue and GALT.
Conflict of interest statement
KB Patterson and ADM Kashuba have received investigator initiated research grants from Merck and GlaxoSmithKline. The remaining authors have no competing interests.
Figures
Similar articles
-
Raltegravir pharmacokinetics in treatment-naive patients is not influenced by race: results from the raltegravir early therapy in African-Americans living with HIV (REAL) study.Antimicrob Agents Chemother. 2013 Feb;57(2):784-8. doi: 10.1128/AAC.01826-12. Epub 2012 Nov 26. Antimicrob Agents Chemother. 2013. PMID: 23183440 Free PMC article. Clinical Trial.
-
Pharmacokinetics of and short-term virologic response to low-dose 400-milligram once-daily raltegravir maintenance therapy.Antimicrob Agents Chemother. 2012 Apr;56(4):1892-8. doi: 10.1128/AAC.05694-11. Epub 2012 Jan 17. Antimicrob Agents Chemother. 2012. PMID: 22252825 Free PMC article. Clinical Trial.
-
Exposure-related effects of atazanavir on the pharmacokinetics of raltegravir in HIV-1-infected patients.Ther Drug Monit. 2010 Dec;32(6):782-6. doi: 10.1097/FTD.0b013e3181fa53b7. Ther Drug Monit. 2010. PMID: 20926993
-
Raltegravir: the first HIV integrase inhibitor.Clin Ther. 2008 Oct;30(10):1747-65. doi: 10.1016/j.clinthera.2008.10.012. Clin Ther. 2008. PMID: 19014832 Review.
-
[Potential of integrase inhibitors to deplete HIV reservoirs or prevent their replenishment].Enferm Infecc Microbiol Clin. 2008 Nov;26 Suppl 12:17-22. doi: 10.1016/s0213-005x(08)76568-6. Enferm Infecc Microbiol Clin. 2008. PMID: 19572421 Review. Spanish.
Cited by
-
A comparison between different anti-retroviral therapy regimes on soluble inflammation markers: a pilot study.AIDS Res Ther. 2020 Oct 14;17(1):61. doi: 10.1186/s12981-020-00316-w. AIDS Res Ther. 2020. PMID: 33054784 Free PMC article.
-
Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment.Front Immunol. 2021 Jun 18;12:670566. doi: 10.3389/fimmu.2021.670566. eCollection 2021. Front Immunol. 2021. PMID: 34220817 Free PMC article. Review.
-
Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy.AIDS. 2016 Jun 19;30(10):1511-20. doi: 10.1097/QAD.0000000000001029. AIDS. 2016. PMID: 26807971 Free PMC article.
-
Comparison of tenofovir plasma and tissue exposure using a population pharmacokinetic model and bootstrap: a simulation study from observed data.J Pharmacokinet Pharmacodyn. 2017 Dec;44(6):631-640. doi: 10.1007/s10928-017-9554-9. Epub 2017 Nov 8. J Pharmacokinet Pharmacodyn. 2017. PMID: 29119381 Free PMC article.
-
Pharmacokinetic/pharmacodynamic investigation of raltegravir with or without lamivudine in the context of HIV-1 pre-exposure prophylaxis (PrEP).J Antimicrob Chemother. 2021 Jul 15;76(8):2129-2136. doi: 10.1093/jac/dkab136. J Antimicrob Chemother. 2021. PMID: 33993302 Free PMC article. Clinical Trial.
References
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338 (13):853–860. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194 (1):11–19. - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278 (5341):1295–1300. - PubMed
-
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278 (5341):1291–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical