Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial
- PMID: 23945571
- PMCID: PMC3814935
- DOI: 10.1097/INF.0b013e31829ff7aa
Simplified antibiotic regimens for the management of clinically diagnosed severe infections in newborns and young infants in first-level facilities in Karachi, Pakistan: study design for an outpatient randomized controlled equivalence trial
Abstract
Background: Infection in young infants is a major cause of morbidity and mortality in low-middle income countries, with high neonatal mortality rates. Timely case management is lifesaving, but the current standard of hospitalization for parenteral antibiotic therapy is not always feasible. Alternative, simpler antibiotic regimens that could be used in outpatient settings have the potential to save thousands of lives.
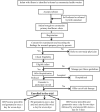
Methods: This trial aims to determine whether 2 simplified antibiotic regimens are equivalent to the reference therapy with 7 days of once-daily (OD) intramuscular (IM) procaine penicillin and gentamicin for outpatient management of young infants with clinically presumed systemic bacterial infection treated in primary health-care clinics in 5 communities in Karachi, Pakistan. The reference regimen is close to the current recommendation of the hospital-based intravenous ampicillin and gentamicin therapy for neonatal sepsis. The 2 comparison arms are (1) IM gentamicin OD and oral amoxicillin twice daily for 7 days; and (2) IM penicillin and gentamicin OD for 2 days, followed by oral amoxicillin twice daily for 5 days; 2250 "evaluable" infants will be enrolled. The primary outcome of this trial is treatment failure (death, deterioration or lack of improvement) within 7 days of enrollment. Results are expected by early 2014.
Discussion: This trial will determine whether simplified antibiotic regimens with fewer injections in combination with high-dose amoxicillin are equivalent to 7 days of IM procaine penicillin and gentamicin in young infants with clinical severe infection. Results will have program and policy implications in countries with limited access to hospital care and high burden of neonatal deaths.
Trial registration: ClinicalTrials.gov NCT01027429.
Figures
References
-
- Lawn JE, Cousens S, Darmstadt GL, et al. Why are 4 million newborn babies dying every year? Lancet. 2004;364:2020. - PubMed
-
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. - PubMed
-
- Darmstadt GL, Black RE, Santosham M. Research priorities and postpartum care strategies for the prevention and optimal management of neonatal infections in less developed countries. Pediatr Infect Dis J. 2000;19:739–750. - PubMed
-
- Zaidi AK, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. - PubMed
-
- World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva, Switzerland: World Health Organization; 2005.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical