Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification
- PMID: 23945934
- PMCID: PMC3814370
- DOI: 10.1093/nar/gkt720
Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification
Abstract
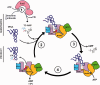
N(6)-threonylcarbamoyladenosine (t(6)A) is a universal tRNA modification essential for normal cell growth and accurate translation. In Archaea and Eukarya, the universal protein Sua5 and the conserved KEOPS/EKC complex together catalyze t(6)A biosynthesis. The KEOPS/EKC complex is composed of Kae1, a universal metalloprotein belonging to the ASHKA superfamily of ATPases; Bud32, an atypical protein kinase and two small proteins, Cgi121 and Pcc1. In this study, we investigated the requirement and functional role of KEOPS/EKC subunits for biosynthesis of t(6)A. We demonstrated that Pcc1, Kae1 and Bud32 form a minimal functional unit, whereas Cgi121 acts as an allosteric regulator. We confirmed that Pcc1 promotes dimerization of the KEOPS/EKC complex and uncovered that together with Kae1, it forms the tRNA binding core of the complex. Kae1 binds l-threonyl-carbamoyl-AMP intermediate in a metal-dependent fashion and transfers the l-threonyl-carbamoyl moiety to substrate tRNA. Surprisingly, we found that Bud32 is regulated by Kae1 and does not function as a protein kinase but as a P-loop ATPase possibly involved in tRNA dissociation. Overall, our data support a mechanistic model in which the final step in the biosynthesis of t(6)A relies on a strictly catalytic component, Kae1, and three partner proteins necessary for dimerization, tRNA binding and regulation.
Figures



References
-
- Ishikura H, Yamada Y, Murao K, Saneyoshi M, Nishimura S. The presence of N-[9-(beta-D-ribofuranosyl)purin-6-ylcarbamoyl]threonine in serine, methionine and lysine transfer RNA’s from Escherichia coli. Biochem. Biophys. Res. Commun. 1969;37:990–995. - PubMed
-
- Powers DM, Peterkofsky A. The presence of N-(purin-6-ylcarbamoyl)threonine in transfer ribonucleic acid species whose codons begin with adenine. J. Biol. Chem. 1972;247:6394–6401. - PubMed
-
- Grosjean H, Sprinzl M, Steinberg S. Posttranscriptionally modified nucleosides in transfer RNA: their locations and frequencies. Biochimie. 1995;77:139–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

