Reversal of neurofibrillary tangles and tau-associated phenotype in the rTgTauEC model of early Alzheimer's disease
- PMID: 23946388
- PMCID: PMC3742920
- DOI: 10.1523/JNEUROSCI.0881-13.2013
Reversal of neurofibrillary tangles and tau-associated phenotype in the rTgTauEC model of early Alzheimer's disease
Abstract
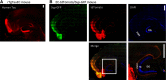
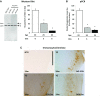
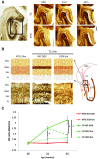
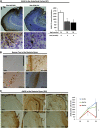
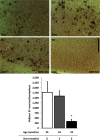
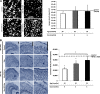
Neurofibrillary tangles (NFTs), a marker of neuronal alterations in Alzheimer's disease (AD) and other tauopathies, are comprised of aggregates of hyperphosphorylated tau protein. We recently studied the formation of NFTs in the entorhinal cortex (EC) and their subsequent propagation through neural circuits in the rTgTauEC mouse model (de Calignon et al., 2012). We now examine the consequences of suppressing transgene expression with doxycycline on the NFT-associated pathological features of neuronal system deafferentation, NFT progression and propagation, and neuronal loss. At 21 months of age we observe that EC axonal lesions are associated with an abnormal sprouting response of acetylcholinesterase (AChE)-positive fibers, a phenotype reminiscent of human AD. At 24 months, NFTs progress, tau inclusions propagate to the dentate gyrus, and neuronal loss is evident. Suppression of the transgene expression from 18 to 24 months led to reversal of AChE sprouting, resolution of Gallyas-positive and Alz50-positive NFTs, and abrogation of progressive neuronal loss. These data suggest that propagation of NFTs, as well as some of the neural system consequences of NFTs, can be reversed in an animal model of NFT-associated toxicity, providing proof in principle that these lesions can be halted, even in established disease.
Figures

References
-
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, Andorfer C, Rosenberry TL, Lewis J, Hutton M, Janus C. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
