CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing
- PMID: 23946390
- PMCID: PMC3742922
- DOI: 10.1523/JNEUROSCI.1224-13.2013
CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing
Abstract
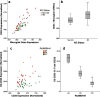
Genome-wide association studies are identifying novel Alzheimer's disease (AD) risk factors. Elucidating the mechanism underlying these polymorphisms is critical to the validation process and, by identifying rate-limiting steps in AD risk, may yield novel therapeutic targets. Here, we elucidate the mechanism of action of the AD-associated polymorphism rs3865444 in the promoter of CD33, a member of the sialic acid-binding Ig-superfamily of lectins (SIGLECs). Immunostaining established that CD33 is expressed in microglia in human brain. Consistent with this finding, CD33 mRNA expression correlated well with expression of the microglial genes CD11b and AIF-1 and was modestly increased with AD status and the rs3865444C AD-risk allele. Analysis of CD33 isoforms identified a common isoform lacking exon 2 (D2-CD33). The proportion of CD33 expressed as D2-CD33 correlated robustly with rs3865444 genotype. Because rs3865444 is in the CD33 promoter region, we sought the functional polymorphism by sequencing CD33 from the promoter through exon 4. We identified a single polymorphism that is coinherited with rs3865444, i.e., rs12459419 in exon 2. Minigene RNA splicing studies in BV2 microglial cells established that rs12459419 is a functional single nucleotide polymorphism (SNP) that modulates exon 2 splicing efficiency. Thus, our primary findings are that CD33 is a microglial mRNA and that rs3865444 is a proxy SNP for rs12459419 that modulates CD33 exon 2 splicing. Exon 2 encodes the CD33 IgV domain that typically mediates sialic acid binding in SIGLEC family members. In summary, these results suggest a novel model wherein SNP-modulated RNA splicing modulates CD33 function and, thereby, AD risk.
Figures


References
-
- Allen M, Zou F, Chai HS, Younkin CS, Crook J, Pankratz VS, Carrasquillo MM, Rowley CN, Nair AA, Middha S, Maharjan S, Nguyen T, Ma L, Malphrus KG, Palusak R, Lincoln S, Bisceglio G, Georgescu C, Schultz D, Rakhshan F, et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012;79:221–228. doi: 10.1212/WNL.0b013e3182605801. - DOI - PMC - PubMed
-
- Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, Alzheimer Disease Neuroimaging I, Morris MC, Evans DA, Johnson K, Sperling RA, Schneider JA, Bennett DA, De Jager PL. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
