Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain
- PMID: 23946395
- PMCID: PMC3742925
- DOI: 10.1523/JNEUROSCI.5286-12.2013
Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain
Abstract
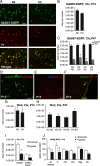
Infants born premature experience hypoxic episodes due to immaturity of their respiratory and central nervous systems. This profoundly affects brain development and results in cognitive impairments. We used a mouse model to examine the impact of hypoxic rearing (9.5-10.5% O2) from postnatal day 3 to 11 (P3-P11) on GABAergic interneurons and the potential for environmental enrichment to ameliorate these developmental abnormalities. At P15 the numbers of cortical interneurons expressing immunohistochemically detectable levels of parvalbumin (PV), somatostatin (SST), and vasoactive intestinal peptide were decreased in hypoxic-reared mice by 59%, 32%, and 38%, respectively, compared with normoxic controls. Hypoxia also decreased total GABA content in frontal neocortex by 31%. However, GAD67-EGFP knock-in mice reared under hypoxic conditions showed no changes in total number of GAD67-EGFP(+) cells and no evidence of increased interneuron death, suggesting that the total number of interneurons was not decreased, but rather, that hypoxic-rearing decreased interneuron marker expression in these cells. In adulthood, PV and SST expression levels were decreased in hypoxic-reared mice. In contrast, intensity of reelin (RLN) expression was significantly increased in adult hypoxic-reared mice compared with normoxic controls. Housing mice in an enriched environment from P21 until adulthood normalized phenotypic interneuron marker expression without affecting total interneuron numbers or leading to increased neurogenesis. Our data show that (1) hypoxia decreases PV and SST and increases RLN expression in cortical interneurons during postnatal cortical development and (2) enriched environment has the capacity to normalize the interneuron abnormalities in cortex.
Figures





References
-
- Bi B, Salmaso N, Komitova M, Simonini MV, Silbereis J, Cheng E, Kim J, Luft S, Ment LR, Horvath TL, Schwartz ML, Vaccarino FM. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
