Lateral entorhinal modulation of piriform cortical activity and fine odor discrimination
- PMID: 23946403
- PMCID: PMC3742931
- DOI: 10.1523/JNEUROSCI.1387-13.2013
Lateral entorhinal modulation of piriform cortical activity and fine odor discrimination
Abstract
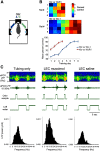
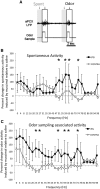
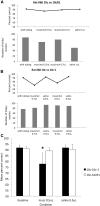
The lateral entorhinal cortex (LEC) receives direct input from olfactory bulb mitral cells and piriform cortical pyramidal cells and is the gateway for olfactory input to the hippocampus. However, the LEC also projects back to the piriform cortex and olfactory bulb. Activity in the LEC is shaped by input from the perirhinal cortices, hippocampus, and amygdala, and thus could provide a rich contextual modulation of cortical odor processing. The present study further explored LEC feedback to anterior piriform cortex by examining how LEC top-down input modulates anterior piriform cortex odor evoked activity in rats. Retrograde viral tracing confirmed rich LEC projections to both the olfactory bulb and piriform cortices. In anesthetized rats, reversible lesions of the ipsilateral LEC increased anterior piriform cortical single-unit spontaneous activity. In awake animals performing an odor discrimination task, unilateral LEC reversible lesions enhanced ipsilateral piriform cortical local field potential oscillations during odor sampling, with minimal impact on contralateral activity. Bilateral LEC reversible lesions impaired discrimination performance on a well learned, difficult odor discrimination task, but had no impact on a well learned simple odor discrimination task. The simple discrimination task was impaired by bilateral reversible lesions of the anterior piriform cortex. Given the known function of LEC in working memory and multisensory integration, these results suggest it may serve as a powerful top-down modulator of olfactory cortical function and odor perception. Furthermore, the results provide potential insight into how neuropathology in the entorhinal cortex could contribute to early olfactory deficits seen in Alzheimer's disease.
Figures





References
-
- Berens P. CircStat: a MATLAB toolbox for circular statistics. J Stat Soft. 2009;31:1–21.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
