Protein kinase Cε is required for spinal analgesic synergy between delta opioid and alpha-2A adrenergic receptor agonist pairs
- PMID: 23946412
- PMCID: PMC3742937
- DOI: 10.1523/JNEUROSCI.4013-12.2013
Protein kinase Cε is required for spinal analgesic synergy between delta opioid and alpha-2A adrenergic receptor agonist pairs
Abstract
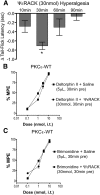
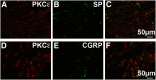
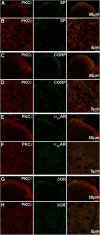
We recently showed that spinal synergistic interactions between δ opioid receptors (δORs) and α2A adrenergic receptors (α2AARs) require protein kinase C (PKC). To identify which PKC isoforms contribute to analgesic synergy, we evaluated the effects of various PKC-isoform-specific peptide inhibitors on synergy between δORs and α2AARs using the tail flick assay of thermal nociception in mice. Only a PKCε inhibitor abolished synergy between a δOR agonist and an α2AAR agonist. We tested a panel of combinations of opioid and adrenergic agonists in PKCε knock-out mice and found that all four combinations of a δOR agonist and an α2AAR agonist required PKCε for antinociceptive synergy. None of the combinations of a μOR agonist with an α2AR agonist required PKCε. Immunohistochemistry confirmed that PKCε could be found in the population of peptidergic primary afferent nociceptors where δORs and α2AARs have been found to extensively colocalize. Immunoreactivity for PKCε was found in the majority of dorsal root ganglion neurons and intensely labeled laminae I and II of the spinal cord dorsal horn. PKCε is widespread in the spinal nociceptive system and in peptidergic primary afferents it appears to be specifically involved in mediating the synergistic interaction between δORs and α2AARs.
Figures



Similar articles
-
Analgesic synergy between opioid and α2 -adrenoceptors.Br J Pharmacol. 2015 Jan;172(2):388-402. doi: 10.1111/bph.12695. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24641506 Free PMC article. Review.
-
Ligand requirements for involvement of PKCε in synergistic analgesic interactions between spinal μ and δ opioid receptors.Br J Pharmacol. 2015 Jan;172(2):642-53. doi: 10.1111/bph.12774. Epub 2014 Nov 24. Br J Pharmacol. 2015. PMID: 24827408 Free PMC article.
-
Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord.J Neurosci. 2009 Oct 21;29(42):13264-73. doi: 10.1523/JNEUROSCI.1907-09.2009. J Neurosci. 2009. PMID: 19846714 Free PMC article.
-
The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy.J Neurosci. 1997 Sep 15;17(18):7157-65. doi: 10.1523/JNEUROSCI.17-18-07157.1997. J Neurosci. 1997. PMID: 9278550 Free PMC article.
-
Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis.Pharmacol Ther. 2009 Aug;123(2):224-38. doi: 10.1016/j.pharmthera.2009.04.001. Epub 2009 Apr 23. Pharmacol Ther. 2009. PMID: 19393691 Free PMC article. Review.
Cited by
-
Morphine Promotes Astrocyte-Preferential Differentiation of Mouse Hippocampal Progenitor Cells via PKCε-Dependent ERK Activation and TRBP Phosphorylation.Stem Cells. 2015 Sep;33(9):2762-72. doi: 10.1002/stem.2055. Epub 2015 Jun 23. Stem Cells. 2015. PMID: 26012717 Free PMC article.
-
Analgesic synergy between opioid and α2 -adrenoceptors.Br J Pharmacol. 2015 Jan;172(2):388-402. doi: 10.1111/bph.12695. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24641506 Free PMC article. Review.
-
The Study of Pain in Rats and Mice.Comp Med. 2019 Dec 1;69(6):555-570. doi: 10.30802/AALAS-CM-19-000062. Epub 2019 Dec 10. Comp Med. 2019. PMID: 31822322 Free PMC article. Review.
-
Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice.Drug Alcohol Depend. 2016 Oct 1;167:190-8. doi: 10.1016/j.drugalcdep.2016.08.017. Epub 2016 Aug 21. Drug Alcohol Depend. 2016. PMID: 27567436 Free PMC article.
-
Ligand requirements for involvement of PKCε in synergistic analgesic interactions between spinal μ and δ opioid receptors.Br J Pharmacol. 2015 Jan;172(2):642-53. doi: 10.1111/bph.12774. Epub 2014 Nov 24. Br J Pharmacol. 2015. PMID: 24827408 Free PMC article.
References
-
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hökfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/S0896-6273(02)01103-0. - DOI - PubMed
-
- Chan AS, Law PY, Loh HH, Ho PN, Wu WM, Chan JS, Wong YH. The first and third intracellular loops together with the carboxy terminal tail of the delta-opioid receptor contribute toward functional interaction with Galpha16. J Neurochem. 2003;87:697–708. doi: 10.1046/j.1471-4159.2003.02040.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
