Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy
- PMID: 23946421
- PMCID: PMC3767549
- DOI: 10.1073/pnas.1303002110
Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy
Abstract
Stapled α-helical peptides have emerged as a promising new modality for a wide range of therapeutic targets. Here, we report a potent and selective dual inhibitor of MDM2 and MDMX, ATSP-7041, which effectively activates the p53 pathway in tumors in vitro and in vivo. Specifically, ATSP-7041 binds both MDM2 and MDMX with nanomolar affinities, shows submicromolar cellular activities in cancer cell lines in the presence of serum, and demonstrates highly specific, on-target mechanism of action. A high resolution (1.7-Å) X-ray crystal structure reveals its molecular interactions with the target protein MDMX, including multiple contacts with key amino acids as well as a role for the hydrocarbon staple itself in target engagement. Most importantly, ATSP-7041 demonstrates robust p53-dependent tumor growth suppression in MDM2/MDMX-overexpressing xenograft cancer models, with a high correlation to on-target pharmacodynamic activity, and possesses favorable pharmacokinetic and tissue distribution properties. Overall, ATSP-7041 demonstrates in vitro and in vivo proof-of-concept that stapled peptides can be developed as therapeutically relevant inhibitors of protein-protein interaction and may offer a viable modality for cancer therapy.
Conflict of interest statement
Conflict of interest statement: All authors are employees of either Aileron Therapeutics, Inc. or Hoffmann-La Roche, Inc. and, in each case, are shareholders of the company for which they work.
Figures


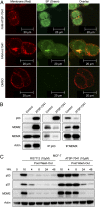
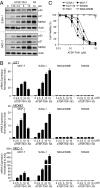
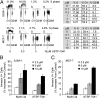
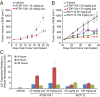

Comment in
-
Anticancer drugs: Stapled peptide reactivates p53.Nat Rev Drug Discov. 2013 Oct;12(10):741. doi: 10.1038/nrd4133. Epub 2013 Sep 20. Nat Rev Drug Discov. 2013. PMID: 24052050 No abstract available.
References
-
- Cheok CF, Verma CS, Baselga J, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8(1):25–37. - PubMed
-
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. - PubMed
-
- Marine JC. MDM2 and MDMX in cancer and development. Curr Top Dev Biol. 2011;94:45–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

