Pioglitazone does not improve insulin signaling in mice with GH over-expression
- PMID: 23946430
- PMCID: PMC3811004
- DOI: 10.1530/JOE-13-0124
Pioglitazone does not improve insulin signaling in mice with GH over-expression
Abstract
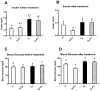
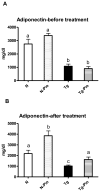
Type 2 diabetes and obesity are very serious health problems in both developed and developing countries. An increased level of GH is known to promote insulin resistance. Transgenic (Tg) mice over-expressing bovine GH are short-living and characterized, among other traits, by hyperinsulinemia and increased insulin resistance in comparison with normal (N) mice. Pioglitazone (PIO) is a member of the thiazolidinediones - a group of insulin-sensitizing drugs that are selective agonists of peroxisome proliferator-activated receptor gamma (PPARγ). The aim of the study was to analyze the effects of PIO on the insulin-signaling pathway in Tg and N mice. Plasma levels of insulin and glucose as well as hepatic levels of proteins involved in insulin signaling were analyzed by ELISA or western blot methods. Treatment with PIO decreased plasma level of glucose in N mice only. Similarly, PIO increased insulin sensitivity (expressed as the relative insulin sensitivity index; RISI) only in N mice. In the liver, PIO decreased the phosphorylation of insulin receptor substrate-1 (IRS1) at a serine residue (Ser(307)-pS-IRS1), which inhibits insulin action, and had a tendency to increase tyrosine phosphorylation of IRS2 (Tyr-pY-IRS2) only in N mice but did not affect either of these parameters in Tg mice. Levels of total and phosphorylated mammalian target of rapamycin were increased in Tg mice. Moreover, the level of AKT2 was decreased by PIO in N mice only. In conclusion, the lack of improvement of insulin sensitivity in insulin-resistant Tg mice during PIO treatment indicates that chronically elevated GH levels can inhibit the beneficial effects of PIO on insulin signaling.
Keywords: growth hormone; insulin signaling; pioglitazone; transgenic mice.
Conflict of interest statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

