Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems
- PMID: 23946468
- PMCID: PMC3820939
- DOI: 10.1074/mcp.M113.030437
Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems
Abstract
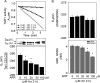
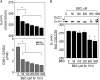
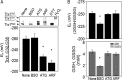
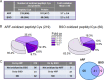
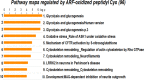
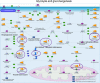
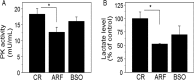
Thioredoxin (Trx) and GSH are the major thiol antioxidants protecting cells from oxidative stress-induced cytotoxicity. Redox states of Trx and GSH have been used as indicators of oxidative stress. Accumulating studies suggest that Trx and GSH redox systems regulate cell signaling and metabolic pathways differently and independently during diverse stressful conditions. In the current study, we used a mass spectrometry-based redox proteomics approach to test responses of the cysteine (Cys) proteome to selective disruption of the Trx- and GSH-dependent systems. Auranofin (ARF) was used to inhibit Trx reductase without detectable oxidation of the GSH/GSSG couple, and buthionine sulfoximine (BSO) was used to deplete GSH without detectable oxidation of Trx1. Results for 606 Cys-containing peptides (peptidyl Cys) showed that 36% were oxidized more than 1.3-fold by ARF, whereas BSO-induced oxidation of peptidyl Cys was only 10%. Mean fold oxidation of these peptides was also higher by ARF than BSO treatment. Analysis of potential functional pathways showed that ARF oxidized peptides associated with glycolysis, cytoskeleton remodeling, translation and cell adhesion. Of 60 peptidyl Cys oxidized due to depletion of GSH, 41 were also oxidized by ARF and included proteins of translation and cell adhesion but not glycolysis or cytoskeletal remodeling. Studies to test functional correlates showed that pyruvate kinase activity and lactate levels were decreased with ARF but not BSO, confirming the effects on glycolysis-associated proteins are sensitive to oxidation by ARF. These data show that the Trx system regulates a broader range of proteins than the GSH system, support distinct function of Trx and GSH in cellular redox control, and show for the first time in mammalian cells selective targeting peptidyl Cys and biological pathways due to deficient function of the Trx system.
Figures

Similar articles
-
Thioredoxin-1 redox signaling regulates cell survival in response to hyperoxia.Free Radic Biol Med. 2014 Oct;75:167-77. doi: 10.1016/j.freeradbiomed.2014.07.023. Epub 2014 Aug 6. Free Radic Biol Med. 2014. PMID: 25106706 Free PMC article.
-
Simultaneous inhibition of glutathione- and thioredoxin-dependent metabolism is necessary to potentiate 17AAG-induced cancer cell killing via oxidative stress.Free Radic Biol Med. 2012 Jan 15;52(2):436-43. doi: 10.1016/j.freeradbiomed.2011.10.493. Epub 2011 Nov 4. Free Radic Biol Med. 2012. PMID: 22100505 Free PMC article.
-
Inhibition of glutathione biosynthesis alters compartmental redox status and the thiol proteome in organogenesis-stage rat conceptuses.Free Radic Biol Med. 2013 Oct;63:325-37. doi: 10.1016/j.freeradbiomed.2013.05.040. Epub 2013 Jun 2. Free Radic Biol Med. 2013. PMID: 23736079 Free PMC article.
-
The thioredoxin antioxidant system.Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review.
-
Redox biology of the intestine.Free Radic Res. 2011 Nov;45(11-12):1245-66. doi: 10.3109/10715762.2011.611509. Epub 2011 Sep 5. Free Radic Res. 2011. PMID: 21831010 Free PMC article. Review.
Cited by
-
Speciation Analysis Highlights the Interactions of Auranofin with the Cytoskeleton Proteins of Lung Cancer Cells.Pharmaceuticals (Basel). 2022 Oct 19;15(10):1285. doi: 10.3390/ph15101285. Pharmaceuticals (Basel). 2022. PMID: 36297397 Free PMC article.
-
Antioxidant enzymes as redox-based biomarkers: a brief review.BMB Rep. 2015 Apr;48(4):200-8. doi: 10.5483/bmbrep.2015.48.4.274. BMB Rep. 2015. PMID: 25560698 Free PMC article. Review.
-
Impact of Atmospheric Microparticles on the Development of Oxidative Stress in Healthy City/Industrial Seaport Residents.Oxid Med Cell Longev. 2015;2015:412173. doi: 10.1155/2015/412173. Epub 2015 Apr 30. Oxid Med Cell Longev. 2015. PMID: 26064419 Free PMC article.
-
Organometallic gold(I) and gold(III) complexes for lung cancer treatment.Front Pharmacol. 2022 Sep 13;13:979951. doi: 10.3389/fphar.2022.979951. eCollection 2022. Front Pharmacol. 2022. PMID: 36176441 Free PMC article. Review.
-
A Direct Comparison of Metabolic Responses to High-Fat Diet in C57BL/6J and C57BL/6NJ Mice.Diabetes. 2016 Nov;65(11):3249-3261. doi: 10.2337/db16-0291. Epub 2016 Aug 5. Diabetes. 2016. PMID: 27495226 Free PMC article.
References
-
- Shiota M., Yokomizo A., Naito S. (2011) Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 51, 1320–1328 - PubMed
-
- Kondo T., Hirose M., Kageyama K. (2009) Roles of oxidative stress and redox regulation in atherosclerosis. J. Atheroscler. Thromb. 16, 532–538 - PubMed
-
- Biguet C., Wakasugi N., Mishal Z., Holmgren A., Chouaib S., Tursz T., Wakasugi H. (1994) Thioredoxin increases the proliferation of human B-cell lines through a protein kinase C-dependent mechanism. J. Biol. Chem. 269, 28865–28870 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

