Biosimilars: part 1: proposed regulatory criteria for FDA approval
- PMID: 23946620
- PMCID: PMC3737980
Biosimilars: part 1: proposed regulatory criteria for FDA approval
Abstract
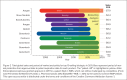
Biosimilars, although not identical to their originator product, are expected to become essential in reducing health care costs and improving access to lifesaving drugs. The FDA must find a way to balance rigorous testing to ensure quality, as is done for generic chemical drugs, with providing a cost-efficient way to expedite approvals of these products.
Figures

References
-
- Hirsch BR, Lyman GH. Biosimilars: Are they ready for primetime in the United States? J Natl Compr Cancer Network. 2011;9:934–943. - PubMed
-
- McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91(3):405–417. - PubMed
-
- Hoffman JM, Li E, Stevenson JG. Preparing for biosimilars: Scientific, regulatory, and practice management issues for pharmacists. Live webcast, 47th ASHP Midyear Clinical Meeting and Exhibition; Las Vegas. December 3, 2012; Available at: www.ashpadvantagemedia.com/downloads/handout_biosimilars.pdf. Accessed February 26, 2013.
LinkOut - more resources
Full Text Sources
