How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth
- PMID: 23947349
- PMCID: PMC3748619
- DOI: 10.1111/imr.12094
How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth
Abstract
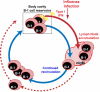
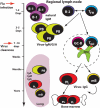
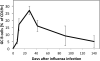
Influenza virus infection induces robust and highly protective B-cell responses. Knowledge gained from the analysis of such protective humoral responses can provide important clues for the design of successful vaccines and vaccination approaches and also provides a window into the regulation of fundamental aspects of B-cell responses that may not be at play when responses to non-replicating agents are studied. Here, I review features of the B-cell response to viruses, with emphasis on influenza virus infection, a highly localized infection of respiratory tract epithelial cells, and a response that is directed against a virus that continuously undergoes genetic changes to its surface spike protein, a major target of neutralizing antibodies. Two aspects of the B-cell response to influenza are discussed here, namely polyreactive natural antibodies and the role and function of germinal center responses. Both these features of the B-cell response raise the question of how important antibody fine-specificity is for long-term protection from infection. As outlined, the pathogenesis of influenza virus and the nature of the antiviral B-cell response seem to emphasize repertoire diversity over affinity maturation as driving forces behind the influenza-specific B-cell immunity.
Keywords: B-1 cells; antibody repertoire; antiviral immunity; extrafollicular foci; germinal centers; plasma cells.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

References
-
- Potter CW. A history of influenza. J Appl Microbiol. 2001;91:572–579. - PubMed
-
- Bos NA, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. - PubMed
-
- Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol. 1997;27:1557–1563. - PubMed
-
- Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14:1127–1130. - PubMed
-
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

