Transcriptional analysis of abdominal fat in genetically fat and lean chickens reveals adipokines, lipogenic genes and a link between hemostasis and leanness
- PMID: 23947536
- PMCID: PMC3765218
- DOI: 10.1186/1471-2164-14-557
Transcriptional analysis of abdominal fat in genetically fat and lean chickens reveals adipokines, lipogenic genes and a link between hemostasis and leanness
Abstract
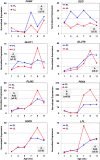
Background: This descriptive study of the abdominal fat transcriptome takes advantage of two experimental lines of meat-type chickens (Gallus domesticus), which were selected over seven generations for a large difference in abdominal (visceral) fatness. At the age of selection (9 wk), the fat line (FL) and lean line (LL) chickens exhibit a 2.5-fold difference in abdominal fat weight, while their feed intake and body weight are similar. These unique avian models were originally created to unravel genetic and endocrine regulation of adiposity and lipogenesis in meat-type chickens. The Del-Mar 14K Chicken Integrated Systems microarray was used for a time-course analysis of gene expression in abdominal fat of FL and LL chickens during juvenile development (1-11 weeks of age).
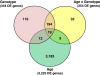
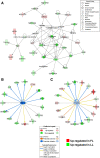
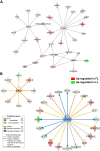
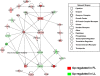
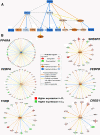
Results: Microarray analysis of abdominal fat in FL and LL chickens revealed 131 differentially expressed (DE) genes (FDR≤0.05) as the main effect of genotype, 254 DE genes as an interaction of age and genotype and 3,195 DE genes (FDR≤0.01) as the main effect of age. The most notable discoveries in the abdominal fat transcriptome were higher expression of many genes involved in blood coagulation in the LL and up-regulation of numerous adipogenic and lipogenic genes in FL chickens. Many of these DE genes belong to pathways controlling the synthesis, metabolism and transport of lipids or endocrine signaling pathways activated by adipokines, retinoid and thyroid hormones.
Conclusions: The present study provides a dynamic view of differential gene transcription in abdominal fat of chickens genetically selected for fatness (FL) or leanness (LL). Remarkably, the LL chickens over-express a large number of hemostatic genes that could be involved in proteolytic processing of adipokines and endocrine factors, which contribute to their higher lipolysis and export of stored lipids. Some of these changes are already present at 1 week of age before the divergence in fatness. In contrast, the FL chickens have enhanced expression of numerous lipogenic genes mainly after onset of divergence, presumably directed by multiple transcription factors. This transcriptional analysis shows that abdominal fat of the chicken serves a dual function as both an endocrine organ and an active metabolic tissue, which could play a more significant role in lipogenesis than previously thought.
Figures



References
-
- Burt DW. Emergence of the chicken as a model organism: implications for agriculture and biology. Poult Sci. 2007;86:1460–1471. - PubMed
-
- Cogburn LA, Porter TE, Duclos MJ, Simon J, Burgess SC, Zhu JJ, Cheng HH, Dodgson JB, Burnside J. Functional genomics of the chicken–a model organism. Poult Sci. 2007;86:2059–2094. - PubMed
-
- Dodgson JB. The chicken genome: some good news and some bad news. Poult Sci. 2007;86:1453–1459. - PubMed
-
- Stern CD. The chick: a great model system becomes even greater. Dev Cell. 2005;8:9–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

