MscS-like mechanosensitive channels in plants and microbes
- PMID: 23947546
- PMCID: PMC3791886
- DOI: 10.1021/bi400804z
MscS-like mechanosensitive channels in plants and microbes
Abstract
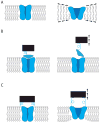
The challenge of osmotic stress is something all living organisms must face as a result of environmental dynamics. Over the past three decades, innovative research and cooperation across disciplines have irrefutably established that cells utilize mechanically gated ion channels to release osmolytes and prevent cell lysis during hypoosmotic stress. Early electrophysiological analysis of the inner membrane of Escherichia coli identified the presence of three distinct mechanosensitive activities. The subsequent discoveries of the genes responsible for two of these activities, the mechanosensitive channels of large (MscL) and small (MscS) conductance, led to the identification of two diverse families of mechanosensitive channels. The latter of these two families, the MscS family, consists of members from bacteria, archaea, fungi, and plants. Genetic and electrophysiological analysis of these family members has provided insight into how organisms use mechanosensitive channels for osmotic regulation in response to changing environmental and developmental circumstances. Furthermore, determining the crystal structure of E. coli MscS and several homologues in several conformational states has contributed to our understanding of the gating mechanisms of these channels. Here we summarize our current knowledge of MscS homologues from all three domains of life and address their structure, proposed physiological functions, electrophysiological behaviors, and topological diversity.
Figures

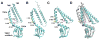

References
-
- Hille B. Ion channels of excitable membranes. 3. Sinauer; Sunderland, Mass: 2001.
-
- Kullmann DM. Neurological channelopathies. Annu Rev Neurosci. 2010;33:151–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

