Dinuclear zinc-ProPhenol-catalyzed enantioselective α-hydroxyacetate aldol reaction with activated ester equivalents
- PMID: 23947595
- PMCID: PMC3812954
- DOI: 10.1021/ol402081p
Dinuclear zinc-ProPhenol-catalyzed enantioselective α-hydroxyacetate aldol reaction with activated ester equivalents
Abstract
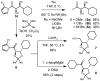
An enantioselective α-hydroxyacetate aldol reaction that employs N-acetyl pyrroles as activated ester equivalents and generates syn 1,2-diols in good yield and diastereoselectivity is reported. This dinuclear zinc-ProPhenol-catalyzed transformation proceeds with high enantioselectivity with a wide variety of substrates including aryl, alyl, and alkenyl aldehydes. The resulting α,β-dihydroxy activated esters are versatile intermediates for the synthesis of a variety of carboxylic acid derivatives including amides, esters, and unsymmetrical ketones.
Figures
Similar articles
-
Development of ProPhenol ligands for the diastereo- and enantioselective synthesis of β-hydroxy-α-amino esters.J Am Chem Soc. 2014 Feb 26;136(8):3016-9. doi: 10.1021/ja4129394. Epub 2014 Feb 13. J Am Chem Soc. 2014. PMID: 24502188 Free PMC article.
-
Generation and tandem reactions of 1-alkenyl-1,1-heterobimetallics: practical and versatile reagents for organic synthesis.J Am Chem Soc. 2008 Mar 19;130(11):3521-31. doi: 10.1021/ja077664u. Epub 2008 Feb 27. J Am Chem Soc. 2008. PMID: 18302376
-
A bidirectional S(E)' strategy for 1,5-syn and 1,5-anti stereocontrol toward the synthesis of complex polyols.Org Lett. 2012 Aug 3;14(15):3866-9. doi: 10.1021/ol3015682. Epub 2012 Jul 19. Org Lett. 2012. PMID: 22813207 Free PMC article.
-
Enantioselective aldol reactions catalyzed by chiral phosphine oxides.Chem Rec. 2013 Aug;13(4):362-70. doi: 10.1002/tcr.201300004. Epub 2013 Jul 4. Chem Rec. 2013. PMID: 23828817 Review.
-
The Catalytic Enantioselective Ugi Four-Component Reactions.Angew Chem Int Ed Engl. 2018 Dec 10;57(50):16266-16268. doi: 10.1002/anie.201811129. Epub 2018 Nov 15. Angew Chem Int Ed Engl. 2018. PMID: 30431219 Free PMC article. Review.
Cited by
-
An Enantioselective Decarboxylative Glycolate Aldol Reaction.Org Lett. 2024 Oct 25;26(42):9040-9045. doi: 10.1021/acs.orglett.4c03251. Epub 2024 Oct 10. Org Lett. 2024. PMID: 39388361 Free PMC article.
-
Catalytic stereoselective Mannich-type reactions for construction of fluorinated compounds.Mol Divers. 2022 Apr;26(2):1267-1310. doi: 10.1007/s11030-021-10235-1. Epub 2021 Jul 6. Mol Divers. 2022. PMID: 34228344 Review.
-
Broad Spectrum Enolate Equivalent for Catalytic Chemo-, Diastereo-, and Enantioselective Addition to N-Boc Imines.J Am Chem Soc. 2015 Dec 23;137(50):15940-6. doi: 10.1021/jacs.5b11248. Epub 2015 Dec 14. J Am Chem Soc. 2015. PMID: 26630114 Free PMC article.
-
Development of ProPhenol ligands for the diastereo- and enantioselective synthesis of β-hydroxy-α-amino esters.J Am Chem Soc. 2014 Feb 26;136(8):3016-9. doi: 10.1021/ja4129394. Epub 2014 Feb 13. J Am Chem Soc. 2014. PMID: 24502188 Free PMC article.
-
Catalytic Asymmetric Mannich Reactions with Fluorinated Aromatic Ketones: Efficient Access to Chiral β-Fluoroamines.Angew Chem Int Ed Engl. 2016 Jan 11;55(2):781-4. doi: 10.1002/anie.201509719. Epub 2015 Nov 26. Angew Chem Int Ed Engl. 2016. PMID: 26609784 Free PMC article.
References
-
-
Mukaiyama T, Shiina I, Uchiro H, Kobayashi S. Bull. Che. Soc. Jpn. 1994;67:1708–1716. (b) For a review of direct aldol reactions, which includes α-hydroxyacetate aldols, see: Trost BM, Brindle CS. Chem. Soc. Rev. 2010;39:1600–1632. (c) For a recent review on additions to imines, which includes α-hydroxycarbonyl enolate donors, see: Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem. Rev. 2011;111:2626–2704.
-
-
-
For selected reviews of osmium-catalyzed syn dihydroxylations, see: Tse MK, Schroeder K, Beller M. In: Modern Oxidation Methods. 2nd edition Bäckvall J-E, editor. Wiley-VCH; Weinheim: 2010. pp. 1–36. Zaitsev AB, Adolfsson H. Synthesis. 2006:1725–1756. Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547.
-
-
- Yoshikawa N, Kumagai N, Matsunaga S, Moll G, Ohshima T, Suzuki T, Shibasaki M. J. Am. Chem. Soc. 2001;123:2466–2467. - PubMed
- Kumagai N, Matsunaga S, Kinoshita T, Harada S, Okada S, Sakamoto S, Yamaguchi K, Shibasaki M. J. Am. Chem. Soc. 2003;125:2169–2178. - PubMed
- Trost BM, Ito H, Silcoff ER. J. Am. Chem. Soc. 2001;123:3367–3368. - PubMed
-
-
Notz W, List B. J. Am. Chem. Soc. 2000;122:7386–7387. For selected recent examples, see the following and references therein: Ma G, Bartoszewicz A, Ibrahem I, Córdova A. Adv. Synth. Catal. 2011;353:3114–3122. Wu C, Fu X, Li S. Tetrahedron: Asymmetry. 2011;22:1063–1073. Chen X-H, Luo S-W, Tang Z, Cun L-F, Mi A-Q, Jiang Y-Z, Gong L-Z. Chem. Eur. J. 2007;13:689–701. Ramasastry SSV, Zhang H, Tanaka F, Barbas CF., III J. Am. Chem. Soc. 2007;129:288–289.
-
-
-
For an enantioselective direct aldol reaction with ester derivatives, see: Misaki T, Takimoto G, Sugimura T. J. Am. Chem. Soc. 2010;132:6286–6287. and references therein.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous