Olfactory bulb and hypothalamic acute-phase responses to influenza virus: effects of immunization
- PMID: 23948712
- PMCID: PMC3874867
- DOI: 10.1159/000351716
Olfactory bulb and hypothalamic acute-phase responses to influenza virus: effects of immunization
Abstract
Background: Within hours of intranasal challenge, mouse-adapted H1N1 A/Puerto Rico/8/34 (PR8) influenza genomic RNA is found in the olfactory bulb (OB) and OB pro-inflammatory cytokines are up-regulated. Severing the olfactory tract delays the acute-phase response (APR) and the APR is attenuated by immunization.
Objectives: To determine if immunization affects OB localization of influenza or the molecular brain mechanisms regulating APR.
Methods: Male mice were immunized with PR8 influenza, then OB viral RNA, APR, and influenza-related cytokine responses were determined after homologous viral challenge.
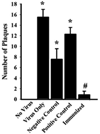
Results: Immunization did not prevent influenza OB viral invasion within 24 h of viral challenge. However, it greatly attenuated OB viral RNA 6 days after viral challenge and the APR including hypothermia and body weight loss responses. Within the OB, 24 h after influenza challenge, prior immunization blocked virus-induced up-regulation of toll-like receptor 7 and interferon (IFN) γ mRNAs. At this time, hypothalamic (HT) growth hormone-releasing hormone receptor and tumor necrosis factor-α mRNAs were greatly enhanced in immunized but not in positive control mice. By 6 days after viral challenge, OB and HT mRNAs returned towards baseline values. In the lung, mRNA up-regulation was greater than that in the brain and maximized 6 days after challenge. Lung IFNγ mRNA decreased at 24 h but increased 6 days after challenge in the positive compared to negative controls. Immunization prevented the up-regulation of most of the flu-related mRNAs measured in lungs.
Conclusion: Collectively, these data suggest a role for OB and HT involvement in immunization protection against influenza infection.
Copyright © 2013 S. Karger AG, Basel.
Figures

References
-
- Oxford JS. Influenza A pandemics of the 20th century with special reference to 1918: virology, pathology and epidemiology. Rev Med Virol. 2000;10:119–133. - PubMed
-
- Fang J, Sanborn CK, Renegar KB, Majde JA, Krueger JM. Influenza viral infections enhance sleep in mice. Proc Soc Exp Biol Med. 1995;210:242–252. - PubMed
-
- Toth LA, Williams R. A quantitative genetic analysis of slow-wave sleep in influenza-infected CXB recombinant inbred mice. Behav Genet. 1999;29:339–348. - PubMed
-
- Conn CA, McClellan JL, Maassab HF, Smitka CW, Majde JA, Kluger MJ. Cytokines and the acute phase response to influenza virus in mice. Am J Physiol. 1995;268:R78–R84. - PubMed
-
- Majde JA, Bohnet SG, Ellis GA, Churchill L, Leyva-Grado V, Wu M, Szentirmai É, Rehman A, Krueger JM. Detection of a mouse-adapted human influenza virus in the olfactory bulb of mice within hours after intranasal infection. J NeuroVirol. 2007;13:399–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

