KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo
- PMID: 23949222
- PMCID: PMC3763441
- DOI: 10.1038/cddis.2013.279
KCa3.1 channels are involved in the infiltrative behavior of glioblastoma in vivo
Abstract
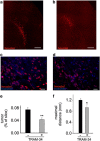
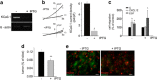
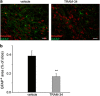
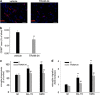
Glioblastoma multiforme (GBM) is a diffuse brain tumor characterized by high infiltration in the brain parenchyma rendering the tumor difficult to eradicate by neurosurgery. Efforts to identify molecular targets involved in the invasive behavior of GBM suggested ion channel inhibition as a promising therapeutic approach. To determine if the Ca(2+)-dependent K(+) channel KCa3.1 could represent a key element for GBM brain infiltration, human GL-15 cells were xenografted into the brain of SCID mice that were then treated with the specific KCa3.1 blocker TRAM-34 (1-((2-chlorophenyl) (diphenyl)methyl)-1H-pyrazole). After 5 weeks of treatment, immunofluorescence analyses of cerebral slices revealed reduced tumor infiltration and astrogliosis surrounding the tumor, compared with untreated mice. Significant reduction of tumor infiltration was also observed in the brain of mice transplanted with KCa3.1-silenced GL-15 cells, indicating a direct effect of TRAM-34 on GBM-expressed KCa3.1 channels. As KCa3.1 channels are also expressed on microglia, we investigated the effects of TRAM-34 on microglia activation in GL-15 transplanted mice and found a reduction of CD68 staining in treated mice. Similar results were observed in vitro where TRAM-34 reduced both phagocytosis and chemotactic activity of primary microglia exposed to GBM-conditioned medium. Taken together, these results indicate that KCa3.1 activity has an important role in GBM invasiveness in vivo and that its inhibition directly affects glioma cell migration and reduces astrocytosis and microglia activation in response to tumor-released factors. KCa3.1 channel inhibition therefore constitutes a potential novel therapeutic approach to reduce GBM spreading into the surrounding tissue.
Figures
References
-
- Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Markovic DS, Glass R, Synowitz M, Nv Rooijen, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64:754–762. - PubMed
-
- Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion-an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27:918–930. - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

