Interaction of formin FH2 with skeletal muscle actin. EPR and DSC studies
- PMID: 23949957
- PMCID: PMC3824300
- DOI: 10.1007/s00249-013-0922-0
Interaction of formin FH2 with skeletal muscle actin. EPR and DSC studies
Abstract
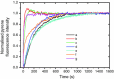
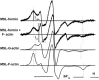
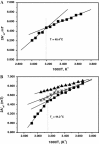
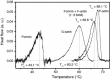
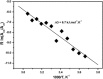
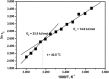
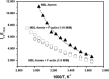
Formins are highly conserved proteins that are essential in the formation and regulation of the actin cytoskeleton. The formin homology 2 (FH2) domain is responsible for actin binding and acts as an important nucleating factor in eukaryotic cells. In this work EPR and DSC were used to investigate the properties of the mDia1-FH2 formin fragment and its interaction with actin. MDia1-FH2 was labeled with a maleimide spin probe (MSL). EPR results suggested that the MSL was attached to a single SH group in the FH2. In DSC and temperature-dependent EPR experiments we observed that mDia1-FH2 has a flexible structure and observed a major temperature-induced conformational change at 41 °C. The results also confirmed the previous observation obtained by fluorescence methods that formin binding can destabilize the structure of actin filaments. In the EPR experiments the intermolecular connection between the monomers of formin dimers proved to be flexible. Considering the complex molecular mechanisms underlying the cellular roles of formins this internal flexibility of the dimers is probably important for manifestation of their biological functions.
Figures
Similar articles
-
Formins regulate actin filament flexibility through long range allosteric interactions.J Biol Chem. 2006 Apr 21;281(16):10727-36. doi: 10.1074/jbc.M510252200. Epub 2006 Feb 20. J Biol Chem. 2006. PMID: 16490788 Free PMC article.
-
Myosin and tropomyosin stabilize the conformation of formin-nucleated actin filaments.J Biol Chem. 2012 Sep 14;287(38):31894-904. doi: 10.1074/jbc.M112.341230. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753415 Free PMC article.
-
Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth.Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13341-6. doi: 10.1073/pnas.0901170106. Epub 2009 Jul 24. Proc Natl Acad Sci U S A. 2009. PMID: 19633191 Free PMC article.
-
Fifteen formins for an actin filament: a molecular view on the regulation of human formins.Biochim Biophys Acta. 2010 Feb;1803(2):152-63. doi: 10.1016/j.bbamcr.2010.01.014. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20102729 Review.
-
Formin-induced nucleation of actin filaments.Curr Opin Cell Biol. 2004 Feb;16(1):99-105. doi: 10.1016/j.ceb.2003.10.019. Curr Opin Cell Biol. 2004. PMID: 15037312 Review.
Cited by
-
Formin' cellular structures: Physiological roles of Diaphanous (Dia) in actin dynamics.Commun Integr Biol. 2013 Nov 1;6(6):e27634. doi: 10.4161/cib.27634. Epub 2014 Jan 8. Commun Integr Biol. 2013. PMID: 24719676 Free PMC article. Review.
-
The role of formin tails in actin nucleation, processive elongation, and filament bundling.J Biol Chem. 2014 Oct 31;289(44):30602-30613. doi: 10.1074/jbc.M114.588368. Epub 2014 Sep 22. J Biol Chem. 2014. PMID: 25246531 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

