Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials
- PMID: 23950195
- PMCID: PMC3805480
- DOI: 10.1136/bmj.f4822
Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials
Abstract
Objectives: To evaluate the efficacy and safety of intravenous iron, focusing primarily on its effects on haemoglobin, requirement for transfusion, and risk of infection.
Design: Systematic review and meta-analysis of randomised controlled trials investigating the safety and efficacy of intravenous iron therapy.
Data sources: Randomised controlled trials from Medline, Embase, and the Cochrane Central Register of Controlled Trials from 1966 to June 2013, with no language restrictions.
Eligibility criteria for selecting studies: Eligible trials were randomised controlled trials of intravenous iron compared with either no iron or oral iron. Crossover and observational studies were excluded.
Main outcome measures: Change in haemoglobin concentration and risk of allogeneic red blood cell transfusion (efficacy) and risk of infection (safety).
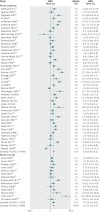
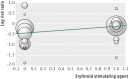
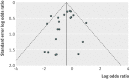
Results: Of the 75 trials meeting the inclusion criteria, 72 studies including 10 605 patients provided quantitative outcome data for meta-analysis. Intravenous iron was associated with an increase in haemoglobin concentration (standardised mean difference 6.5 g/L, 95% confidence interval 5.1 g/L to 7.9 g/L) and a reduced risk of requirement for red blood cell transfusion (risk ratio 0.74, 95% confidence interval 0.62 to 0.88), especially when intravenous iron was used with erythroid stimulating agents (ESAs) or in patients with a lower baseline plasma ferritin concentration. There were no significant interactions between the efficacy of intravenous iron and type or dose administered. Intravenous iron was, however, associated with a significant increase in risk of infection (relative risk 1.33, 95% confidence interval 1.10 to 1.64) compared with oral or no iron supplementation. The results remained similar when only high quality trials were analysed.
Conclusions: Intravenous iron therapy is effective in increasing haemoglobin concentration and reducing the risk of allogeneic red blood cell transfusion and could have broad applicability to a range of acute care settings. This potential benefit is counterbalanced by a potential increased risk of infection.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures



Comment in
-
Intravenous iron therapy for treatment of anaemia.BMJ. 2013 Sep 3;347:f5378. doi: 10.1136/bmj.f5378. BMJ. 2013. PMID: 24002811 No abstract available.
References
-
- Mclean E. Worldwide prevalence of anaemia 1993-2005. World Health Organization, 2008.
-
- Isbister JP, Shander A, Spahn DR, Erhard J, Farmer SL, Hofmann A. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev 2011;25:89-101. - PubMed
-
- Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoeisis. Transfusion 2012;52:1584-92. - PubMed
-
- KDOQI, National Kidney Foundation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis 2006;47(5 suppl 3):S16-85. - PubMed
-
- Hofmann A, Farmer S, Towler SC. Strategies to preempt and reduce the use of blood products: an Australian perspective. Curr Opin Anaesthesiol 2012;25:66-73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
