Structure of the YajR transporter suggests a transport mechanism based on the conserved motif A
- PMID: 23950222
- PMCID: PMC3767500
- DOI: 10.1073/pnas.1308127110
Structure of the YajR transporter suggests a transport mechanism based on the conserved motif A
Abstract
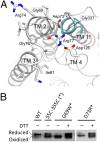
The major facilitator superfamily (MFS) is the largest family of secondary active transporters and is present in all life kingdoms. Detailed structural basis of the substrate transport and energy-coupling mechanisms of these proteins remain to be elucidated. YajR is a putative proton-driven MFS transporter found in many Gram-negative bacteria. Here we report the crystal structure of Escherichia coli YajR at 3.15 Å resolution in an outward-facing conformation. In addition to having the 12 canonical transmembrane helices, the YajR structure includes a unique 65-residue C-terminal domain which is independently stable. The structure is unique in illustrating the functional role of "sequence motif A." This highly conserved element is seen to stabilize the outward conformation of YajR and suggests a general mechanism for the conformational change between the inward and outward states of the MFS transporters.
Keywords: charge relay; charge-dipole interaction; membrane potential; protonation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Marger MD, Saier MH., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993;18(1):13–20. - PubMed
-
- Saier MH, Jr, et al. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1(2):257–279. - PubMed
-
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

