The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms
- PMID: 23950711
- PMCID: PMC3738486
- DOI: 10.1371/journal.ppat.1003526
The extracellular matrix Component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms
Abstract
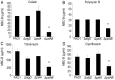
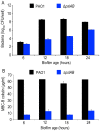
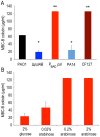
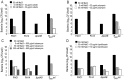
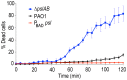
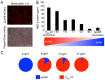
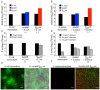
Bacteria within biofilms secrete and surround themselves with an extracellular matrix, which serves as a first line of defense against antibiotic attack. Polysaccharides constitute major elements of the biofilm matrix and are implied in surface adhesion and biofilm organization, but their contributions to the resistance properties of biofilms remain largely elusive. Using a combination of static and continuous-flow biofilm experiments we show that Psl, one major polysaccharide in the Pseudomonas aeruginosa biofilm matrix, provides a generic first line of defense toward antibiotics with diverse biochemical properties during the initial stages of biofilm development. Furthermore, we show with mixed-strain experiments that antibiotic-sensitive "non-producing" cells lacking Psl can gain tolerance by integrating into Psl-containing biofilms. However, non-producers dilute the protective capacity of the matrix and hence, excessive incorporation can result in the collapse of resistance of the entire community. Our data also reveal that Psl mediated protection is extendible to E. coli and S. aureus in co-culture biofilms. Together, our study shows that Psl represents a critical first bottleneck to the antibiotic attack of a biofilm community early in biofilm development.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Flemming HC, Wingender J (2010) The biofilm matrix. Nature reviews Microbiology 8: 623–633. - PubMed
-
- Branda SS, Vik S, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends in microbiology 13: 20–26. - PubMed
-
- Pieters RJ (2011) Carbohydrate mediated bacterial adhesion. Advances in experimental medicine and biology 715: 227–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

