Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export
- PMID: 23950716
- PMCID: PMC3738491
- DOI: 10.1371/journal.ppat.1003546
Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export
Abstract
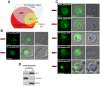
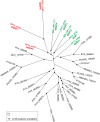
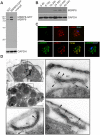
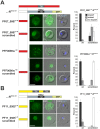
Malaria blood stage parasites export a large number of proteins into their host erythrocyte to change it from a container of predominantly hemoglobin optimized for the transport of oxygen into a niche for parasite propagation. To understand this process, it is crucial to know which parasite proteins are exported into the host cell. This has been aided by the PEXEL/HT sequence, a five-residue motif found in many exported proteins, leading to the prediction of the exportome. However, several PEXEL/HT negative exported proteins (PNEPs) indicate that this exportome is incomplete and it remains unknown if and how many further PNEPs exist. Here we report the identification of new PNEPs in the most virulent malaria parasite Plasmodium falciparum. This includes proteins with a domain structure deviating from previously known PNEPs and indicates that PNEPs are not a rare exception. Unexpectedly, this included members of the MSP-7 related protein (MSRP) family, suggesting unanticipated functions of MSRPs. Analyzing regions mediating export of selected new PNEPs, we show that the first 20 amino acids of PNEPs without a classical N-terminal signal peptide are sufficient to promote export of a reporter, confirming the concept that this is a shared property of all PNEPs of this type. Moreover, we took advantage of newly found soluble PNEPs to show that this type of exported protein requires unfolding to move from the parasitophorous vacuole (PV) into the host cell. This indicates that soluble PNEPs, like PEXEL/HT proteins, are exported by translocation across the PV membrane (PVM), highlighting protein translocation in the parasite periphery as a general means in protein export of malaria parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- World Health Organization (2011) World Malaria Report 2011. http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf.
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415: 673–679. - PubMed
-
- Bannister LH, Hopkins JM, Fowler RE, Krishna S, Mitchell GH (2000) A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol Today 16: 427–433. - PubMed
-
- Grüring C, Heiber A, Kruse F, Ungefehr J, Gilberger TW, Spielmann T (2011) Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat Commun 2: 165. - PubMed
-
- Maier AG, Cooke BM, Cowman AF, Tilley L (2009) Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol 7: 341–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

