miR-105 inhibits prostate tumour growth by suppressing CDK6 levels
- PMID: 23950948
- PMCID: PMC3737265
- DOI: 10.1371/journal.pone.0070515
miR-105 inhibits prostate tumour growth by suppressing CDK6 levels
Abstract
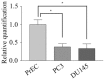
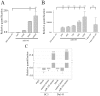
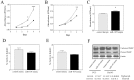
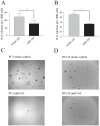
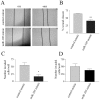
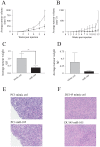
A significant role for micro (mi)RNA in the regulation of gene expression in tumours has been recently established. In order to further understand how miRNA expression may contribute to prostate tumour growth and progression, we evaluated expression of miRNA in two invasive prostate tumour lines, PC3 and DU145, and compared it to that in normal prostate epithelial cells. Although a number of miRNAs were differentially expressed, we focused our analysis on miR-105, a novel miRNA not previously linked to prostate cancer. miR-105 levels were significantly decreased in both tumour cell lines in comparison to normal prostate epithelial cells. To determine its potential role in prostate cancer pathogenesis, we overexpressed miR-105 in both PC3 and DU145 cells and determined its effect on various tumourigenic properties. miR-105 overexpression inhibited tumour cell proliferation, tumour growth in anchorage-independent three-dimensional conditions and tumour invasion in vitro, properties of highly aggressive tumour cells. Of potential clinical significance, miR-105 overexpression inhibited tumour growth in vivo in xenograft models using these cell lines. We further identified CDK6 as a putative target of miR-105 which is likely a main contributor to the inhibition of tumour cell growth observed in our assays. Our results suggest that miR-105 inhibits tumour cell proliferation and hence may represent a novel therapeutically relevant cellular target to inhibit tumour growth or a marker of aggressive tumours in prostate cancer patients.
Conflict of interest statement
Figures

References
-
- Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854. - PubMed
-
- Wightman B, Ha I, Ruvkun G (1993) Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855–862. - PubMed
-
- Winter J, Jung S, Keller S, Gregory RI, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234. - PubMed
-
- Lee Y, Ahn C, Han J, Choi H, Kim J, et al. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419. - PubMed
-
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U (2004) Nuclear export of microRNA precursors. Science 303: 95–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

