Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice
- PMID: 23950972
- PMCID: PMC3738549
- DOI: 10.1371/journal.pone.0070618
Acute toxicity of intravenously administered titanium dioxide nanoparticles in mice
Abstract
Background: With a wide range of applications, titanium dioxide (TiO₂) nanoparticles (NPs) are manufactured worldwide in large quantities. Recently, in the field of nanomedicine, intravenous injection of TiO₂ nanoparticulate carriers directly into the bloodstream has raised public concerns on their toxicity to humans.
Methods: In this study, mice were injected intravenously with a single dose of TiO₂ NPs at varying dose levels (0, 140, 300, 645, or 1387 mg/kg). Animal mortality, blood biochemistry, hematology, genotoxicity and histopathology were investigated 14 days after treatment.
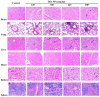
Results: Death of mice in the highest dose (1387 mg/kg) group was observed at day two after TiO₂ NPs injection. At day 7, acute toxicity symptoms, such as decreased physical activity and decreased intake of food and water, were observed in the highest dose group. Hematological analysis and the micronucleus test showed no significant acute hematological or genetic toxicity except an increase in the white blood cell (WBC) count among mice 645 mg/kg dose group. However, the spleen of the mice showed significantly higher tissue weight/body weight (BW) coefficients, and lower liver and kidney coefficients in the TiO₂ NPs treated mice compared to control. The biochemical parameters and histological tissue sections indicated that TiO₂ NPs treatment could induce different degrees of damage in the brain, lung, spleen, liver and kidneys. However, no pathological effects were observed in the heart in TiO₂ NPs treated mice.
Conclusions: Intravenous injection of TiO₂ NPs at high doses in mice could cause acute toxicity effects in the brain, lung, spleen, liver, and kidney. No significant hematological or genetic toxicity was observed.
Conflict of interest statement
Figures



References
-
- Kisin ER, Murray AR, Keane MJ, Shi XC, Schwegler-Berry D, et al. (2007) Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health A 70: 2071–2079. - PubMed
-
- Robertson TA, Sanchez WY, Roberts MS (2010) Are commercially available nanoparticles safe when applied to the skin? J Biomed Nanotechnol 6: 452–468. - PubMed
-
- Iavicoli I, Leso V, Fontana L, Bergamaschi A (2011) Toxicological effects of titanium dioxide nanoparticles: a review of in vitro mammalian studies. Eur Rev Med Pharmacol Sci 15: 481–508. - PubMed
-
- Zhao J, Bowman L, Zhang X, Vallyathan V, Young SH, et al. (2009) Titanium dioxide (TiO2) nanoparticles induce JB6 cell apoptosis through activation of the caspase-8/Bid and mitochondrial pathways. J Toxicol Environ Health A 72: 1141–1149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

