Trypsin potentiates human fibrocyte differentiation
- PMID: 23951012
- PMCID: PMC3737277
- DOI: 10.1371/journal.pone.0070795
Trypsin potentiates human fibrocyte differentiation
Abstract
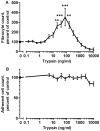
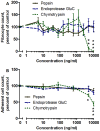
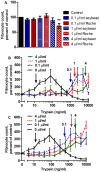
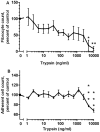
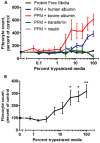
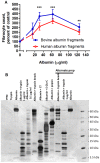
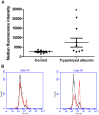
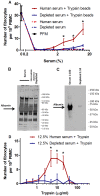
Trypsin-containing topical treatments can be used to speed wound healing, although the mechanism of action is unknown. To help form granulation tissue and heal wounds, monocytes leave the circulation, enter the wound tissue, and differentiate into fibroblast-like cells called fibrocytes. We find that 20 to 200 ng/ml trypsin (concentrations similar to those used in wound dressings) potentiates the differentiation of human monocytes to fibrocytes in cell culture. Adding trypsin inhibitors increases the amount of trypsin needed to potentiate fibrocyte differentiation, suggesting that the potentiating effect is dependent on trypsin proteolytic activity. Proteases with other site specificities such as pepsin, endoprotease GluC, and chymotrypsin do not potentiate fibrocyte differentiation. This potentiation requires the presence of albumin in the culture medium, and tryptic fragments of human or bovine albumin also potentiate fibrocyte differentiation. These results suggest that topical trypsin speeds wound healing by generating tryptic fragments of albumin, which in turn potentiate fibrocyte differentiation.
Conflict of interest statement
Figures
Similar articles
-
A brief exposure to tryptase or thrombin potentiates fibrocyte differentiation in the presence of serum or serum amyloid p.J Immunol. 2015 Jan 1;194(1):142-50. doi: 10.4049/jimmunol.1401777. Epub 2014 Nov 26. J Immunol. 2015. PMID: 25429068 Free PMC article.
-
TNF-α-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation.Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):11929-34. doi: 10.1073/pnas.1507387112. Epub 2015 Sep 8. Proc Natl Acad Sci U S A. 2015. PMID: 26351669 Free PMC article.
-
High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation.PLoS One. 2011;6(10):e26078. doi: 10.1371/journal.pone.0026078. Epub 2011 Oct 11. PLoS One. 2011. PMID: 22022512 Free PMC article.
-
Fibrocytes in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1224:79-85. doi: 10.1007/978-3-030-35723-8_6. Adv Exp Med Biol. 2020. PMID: 32036606 Free PMC article. Review.
-
Fibrocytes, Wound Healing, and Corneal Fibrosis.Invest Ophthalmol Vis Sci. 2020 Feb 7;61(2):28. doi: 10.1167/iovs.61.2.28. Invest Ophthalmol Vis Sci. 2020. PMID: 32084275 Free PMC article. Review.
Cited by
-
A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting.Int J Parasitol. 2014 May;44(6):369-79. doi: 10.1016/j.ijpara.2014.01.010. Epub 2014 Feb 28. Int J Parasitol. 2014. PMID: 24583183 Free PMC article.
-
The Role of Trypsin:Chymotrypsin in Tissue Repair.Adv Ther. 2018 Jan;35(1):31-42. doi: 10.1007/s12325-017-0648-y. Epub 2017 Dec 5. Adv Ther. 2018. PMID: 29209994 Free PMC article. Review.
-
A brief exposure to tryptase or thrombin potentiates fibrocyte differentiation in the presence of serum or serum amyloid p.J Immunol. 2015 Jan 1;194(1):142-50. doi: 10.4049/jimmunol.1401777. Epub 2014 Nov 26. J Immunol. 2015. PMID: 25429068 Free PMC article.
-
The long pentraxin PTX3 promotes fibrocyte differentiation.PLoS One. 2015 Mar 16;10(3):e0119709. doi: 10.1371/journal.pone.0119709. eCollection 2015. PLoS One. 2015. PMID: 25774777 Free PMC article.
-
TNF-α-stimulated fibroblasts secrete lumican to promote fibrocyte differentiation.Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):11929-34. doi: 10.1073/pnas.1507387112. Epub 2015 Sep 8. Proc Natl Acad Sci U S A. 2015. PMID: 26351669 Free PMC article.
References
-
- Vowden KR, Vowden P (2009) The prevalence, management and outcome for acute wounds identified in a wound care survey within one English health care district. J Tissue Viability 18: 7–12. - PubMed
-
- Vowden KR, Vowden P (2009) A survey of wound care provision within one English health care district. J Tissue Viability 18: 2–6. - PubMed
-
- Srinivasaiah N, Dugdall H, Barrett S, Drew PJ (2007) A point prevalence survey of wounds in north-east England. J Wound Care 16: 413–416, 418–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

